The allogeneic transfusion-related immunomodulation (TRIM) may be responsible for an increase in survival of renal transplants but in contrast it could increase the rate of bacterial infections or the recurrence rate of tumors post-operatively.
ObjectiveThis review focuses in the implications of perioperative allogeneic transfusions on the immune-inflammatory response of surgical transfused patients.
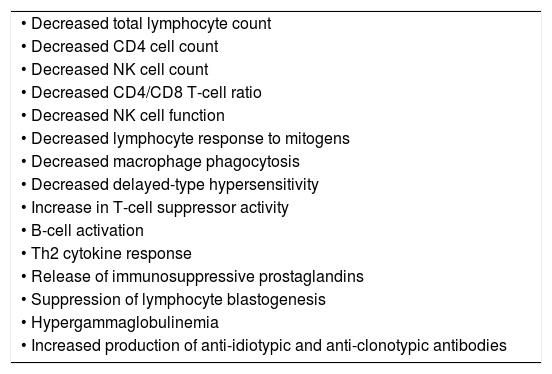
ResultsABTs modify immune functions in recipients including decrease of the number of lymphocytes; decrease the CD4 cells; decrease the CD4/CD8 T-cell ratio; decrease NK cells; and decrease the lymphocyte response to mitogens. TRIM effects may be mediated by allogeneic white cells present in blood products; soluble peptides present in transfused plasma; and/or biologic mediators released into the supernatant of blood units. A recent systematic review and meta-analysis including 36 clinical observational studies (n=174,036) concluded that perioperative ABTs not only decreased overall survival and reduced colorectal cancer-specific survival. Furthermore ABTs increased the rate of infectious, cardiac, pulmonary and anastomotic complications in colorectal cancer patients undergoing surgery.
ConclusionsIt has been demonstrated by laboratory tests that TRIM is associated with transfusion recipient immune alterations but its influence in colorectal cancer recurrence after resection remains controversial though may exist. Surgical techniques reducing intraoperative blood loss have limited the number of ABTs perioperatively, however increase in mortality continues to be reported in literature after ABT in colorectal cancer surgery. Poor survival associated to TRIM in colorectal cancer might be due to higher number of allogeneic transfused units and/or prolonged length of blood storage.
Colorectal cancer is one of most common type of cancer in the United States and the 2nd to 4th in most nations around the globe. In 2017, an estimated 135,430 people will be diagnosed with colorectal cancer, almost 50% will die from this cancer which is increasing among young adults.1 Allogeneic blood transfusions (ABTs) are commonly considered during the perioperative period due either to previous anemia (chronic bleeding from the growth) or intraoperative bleeding.2 Although anemia is an independent risk factor for complications and longer hospital stay after colectomy, the need and time for ABT is controversial. Perioperative ABT has been associated to an increased rate of postoperative infections, recurrence of the disease, and also to a poor survival in colorectal cancer.2,3 Moreover, ABT significantly increase some adverse risks in recipients including hemolytic and non-hemolytic transfusion reactions, graft-versus-host disease, transfusion-related acute lung injury (TRALI), and transmission of infectious agents.4 ABT have also been associated to several host immune function alterations. The lingering question is whether these laboratory immune alterations reflect negative clinically relevant consequences in the recipient's immune vigilance. The group of immunomodulatory and pro-inflammatory effects attributable to ABT are reported in the literature as transfusion-related immunomodulation (TRIM).4,5
The immunomodulatory effects of ABT might be beneficial for recipients of kidney allografts prolonging the graft survival, in reducing the relapse rate in patients with Crohn's disease, and in reducing the rate of recurrence of spontaneous abortion in pregnant women; however these ABT-associated immunomodulatory and pro-inflammatory effects might adversely affect overall prognosis in patients with a malignancy who undergo curative cancer surgery, and/or increase the risk for postoperative bacterial infections. In this paper we aimed to review the current opinion and new data on the proposed TRIM mechanisms and the effects of perioperative ABT in colorectal cancer on postoperative infections and long-time survival.
The normal immune responseIn order to initiate a normal immune response, T cells must recognize alloantigens associated with the MHC (HLA) complex. The direct allorecognition pathway, that is the strongest stimulator of immunity, occurs when recipient undifferentiated naïve T-helper cells directly interact with Class II molecules encoded by the MHC on donor antigen-presenting-cells (APCs); while the indirect allorecognition, that is about 100-fold less potent in inducing alloimmunity, occurs when allelic donor antigens are processed and presented by recipient APCs to recipient undifferentiated naïve T-helper cells. The alloantigen-T cell receptor interaction provides the first signal to cytokine expression, while the necessary co-stimulatory second signal is delivered through the CD28 and the CTLA-4 T-cell receptors which regulate interleukin secretion. The various released cytokines cause the proliferation and differentiation of alloantigen-specific T cells. Depending on the profile of the cytokine secretion these naïve T cells can differentiate into Th1 or Th2 cells (Th1/Th2 paradigm).3 Th1 cells produce the pro-inflammatory cytokines interferon-γ, IL-2 and TNF-β, and are related to cellular immune response against intracellular pathogens, cancer cells, and the stimulation of delayed-type hypersensitivity. In opposition, Th2 cells secrete the anti-inflammatory cytokines IL-4, IL-5, IL-10 and IL-13 which drive the Th2 pathway toward humoral immunity involved in allergic reaction and B cells antibody production directed against extracellular organisms.5 Type-2 immune response also contributes to the tolerance of allografts and of the fetus during pregnancy. Considering that ABT results in exposure to alloantigens it is immunogenic and may elicit a primary immune response resulting in the formation of alloantibodies (Th1 cellular immune response). However, ABT may paradoxically at the same time suppress immune function (Th2 humoral immune response). Over the past few years new lymphocyte subsets have been identified such as T regulatory (Treg) cells, Th3 and Th17 representing strongly-differentiated effector T cells.5,6 Since Treg cells maintain normal immune self-tolerance their dysfunction may cause autoimmune disease and severe allergy.4
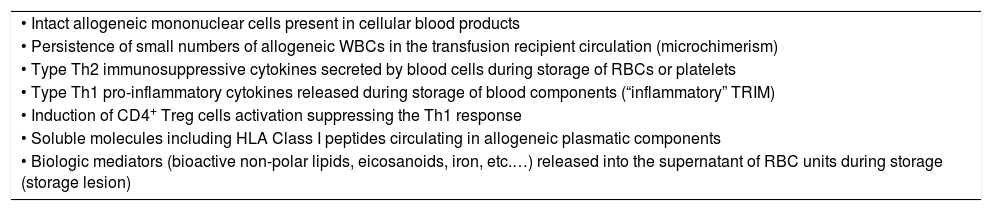
Mechanisms of TRIMThe donor and recipient mechanisms responsible for mediating alloimmunity and immunomodulation following ABT are complex and probably multifactorial. Although the exact mechanism of TRIM has not yet been fully elucidated it has been postulated that the TRIM effects are immunologically mediated. ABTs have been shown to modify several immune functions in recipients such as: decrease of the total number of lymphocytes; decrease the number of CD4 cells, decrease the CD4/CD8 T-cell ratio, decrease the absolute number of NK cells, decrease the lymphocyte response to mitogens (Table 1).7 It has been suggested that TRIM effects may be mediated by: (1) allogeneic WBCs present in blood products; (2) soluble molecules including HLA Class I peptides present in transfused allogeneic plasma; and/or (3) biologic mediators released into the supernatant of red cells (RBCs) or platelet concentrates (Table 2).8
Allogeneic blood transfusion-associated immune function alterations in the transfusion recipient.
| • Decreased total lymphocyte count |
| • Decreased CD4 cell count |
| • Decreased NK cell count |
| • Decreased CD4/CD8 T-cell ratio |
| • Decreased NK cell function |
| • Decreased lymphocyte response to mitogens |
| • Decreased macrophage phagocytosis |
| • Decreased delayed-type hypersensitivity |
| • Increase in T-cell suppressor activity |
| • B-cell activation |
| • Th2 cytokine response |
| • Release of immunosuppressive prostaglandins |
| • Suppression of lymphocyte blastogenesis |
| • Hypergammaglobulinemia |
| • Increased production of anti-idiotypic and anti-clonotypic antibodies |
Proposed mechanisms of transfusion-related immunomodulation (TRIM).
| • Intact allogeneic mononuclear cells present in cellular blood products |
| • Persistence of small numbers of allogeneic WBCs in the transfusion recipient circulation (microchimerism) |
| • Type Th2 immunosuppressive cytokines secreted by blood cells during storage of RBCs or platelets |
| • Type Th1 pro-inflammatory cytokines released during storage of blood components (“inflammatory” TRIM) |
| • Induction of CD4+ Treg cells activation suppressing the Th1 response |
| • Soluble molecules including HLA Class I peptides circulating in allogeneic plasmatic components |
| • Biologic mediators (bioactive non-polar lipids, eicosanoids, iron, etc.…) released into the supernatant of RBC units during storage (storage lesion) |
Allogeneic WBCs present in transfused cellular blood products have been implicated in various transfusion reactions including febrile transfusion reactions, HLA alloimmunization and transfusion platelet refractoriness, TRALI, and transfusion infections in humans.9 Data from animal models have also shown that transfusion of intact allogeneic WBCs are associated with deleterious immunosuppressive effects in the recipient. Experiments with inbred (mice) and outbred (rabbits) animals indicated that ABTs may enhance the growth of animal tumors and that this effect is ameliorated by pre-storage (but not by post-storage) leukoreduction of the allogeneic blood.10 Animals with either non-established or established tumors transfused with non-leukoreduced ABT showed a significantly higher numbers of pulmonary tumors than animals transfused with leukoreduced ABT.10 In this context, humoral immune tolerance was also observed in mice infused with donor UV-B irradiated WBCs free of plasma and platelets.11
Blood product leukoreduction of a 3 log (99.9%) magnitude with the use of third-generation filters has been reported to reduce the rate of ABT-associated alloimmunization.9 More recently, it was confirmed that leukemic patients transfused with leukoreduced or UV-treated blood products not only generated a significant lower rates of both Class I and Class II HLA antibodies compared to those receiving non-leukoreduced blood, but also that both strategies resulted in shorter persistence of circulating Class I HLA antibodies.12
The production of the TRIM effect may require donor APCs expressing the alloantigen and the CD200 co-stimulatory molecule that is a transmembrane protein of the immunoglobulin superfamily. The interaction between donor's CD200 and its receptor on the recipient's T cells culminates in the release of TGF-β, a Th-2 cytokine associated to immune tolerance.13 It has been suggested that during blood storage WBCs lose their immunogenicity, therefore transfusion of allogeneic RBCs containing WBCs stored for prolonged periods of time could be associated with the induction of T-cell anergy and blood recipient immunosuppression.6,9
The persistence of small numbers of HLA compatible allogeneic WBCs in the transfusion recipient circulation, referred to as microchimerism has also been proposed as a possible mechanism of TRIM because such long-term cell engraftment could down regulate the recipient's immune response facilitating tolerance of blood donor alloantigens. Transfusion-associated microchimerism has been detected in allogeneically transfused trauma patients at hospital discharge, and the cell chimerism may increase over months to years in about 10% of the patients.14 Interestingly, it has been reported that transfusion-associated microchimerism occurs even after prestorage leukoreduced ABT, it is not dose dependent, and may be induced by transfusion of RBC units stored for more than 3 weeks.15 Transfusion after severe injury resulted in microchimerism that lasts for up to 60 years in about 10% of combat-injured US veterans.16 In addition, microchimerism of donor WBCs was described to be associated with secretion of Th2 cytokines (IL-4, IL-10 and TGF-β) which suppress allograft rejection in transfused orthopedic patients.17
Although TRIM has usually been associated with the presence of WBCs in the transfused blood component, animal studies performed to compare graft rejection in non-transfused mice to mice transfused with leukoreduced allogeneic fresh platelets showed that fresh platelets can induce TRIM independently of WBCs because of their MHC Class I antigen expression.18
TRIM mediated by soluble moleculesSoluble HLA (sHLA) molecules are normally found in the plasma of healthy individuals, but they are present in higher levels in the plasma of patients with immune, infectious or inflammatory conditions. Although the biologic significance of these molecules is not fully elucidated, it has been suggested that the infusion of large amounts of soluble antigens may be a possible cause of TRIM. In this context it has been shown that HLA Class I peptides may induce antigen-specific immunomodulation since transfused allogeneic blood products containing plasmatic sHLA peptides could provoke thymic clonal deletion of T cells directed against donor alloantigens.19
The infusion of soluble Fas-ligand (sFasL) present in the supernatant of plasma of stored RBC or platelet units may impair the function of the recipient's cytotoxic T and NK cells involved in the apoptosis of virus-infected cells.20 Data from an animal model showed that non-leukoreduced ABT up-regulates the expression of Fas/FasL on spleen T cells of transfused mice and promotes their apoptosis ultimately causing clonal deletion.21 More recently, ex vivo and in vitro experiments suggested that the down regulation of NK-cell activity observed early after ABT is mediated not only by Class I sHLA but also by sFasL and TGF-β1.22 Furthermore, patients chronically transfused with post-storage leukoreduced RBCs showed larger amounts of soluble CD8 molecules capable of binding membrane and sHLA-I molecules than patients transfused with pre-storage leukoreduced RBCs suggesting that soluble CD8 may act as a modulator of the sHLA-I mediated TRIM.23
TRIM mediated by biologic mediatorsPre-storage leukoreduction might avoid the accumulation of bioactive mediators released by WBCs during storage of blood products. Deteriorating WBCs present in stored blood units may release enzymes from intracellular granules which act on RBCs membrane to produce bioactive lipids that activate endothelial cells and prime neutrophils enhancing cytotoxicity and production of superoxide. Histamine, eosinophil protein X, myeloperoxidase, eosinophil cationic protein, and plasminogen activator inhibitor-1 which may cause tissue damage have been shown to be increased in the supernatant of stored RBC units.24 Such reported biologic alterations in recipients may reflect adverse pro-inflammatory transfusion effects rather than immunosuppressive effects.
The levels of biologically active cytokines, predominantly Th2 cytokines are increased in non-leukoreduced RBC concentrates.25 Furthermore, the supernatant of leukoreduced stored packed RBCs induce activation of CD4+ Treg cells that coexpress IL-2 receptor, inhibit IL-2 production suppressing the Th1 response.24 Transfusion of allogeneic unmodified non-leukoreduced RBC units to orthopedic patients is associated with early postoperative lymphopenia, decrease in the number of NK cells, and an increase of CD4+CD25−CD69+ and CD4+GITR+ subsets of Tregcells.26,27
The mechanisms responsible for the progressive reduction of RBC viability over the blood component storage period have not yet been completely elucidated. Nonetheless, it has been suggested that transfusion of older stored RBCs produces an intense inflammatory and/or immunosuppressive response and may increase the risk of infections and multiorgan failure in critically hospitalized patients.3 In vitro studies demonstrated that RBC units supernatant induced suppressive Tregs that inhibited proliferation of T-responder cells, and that the induction was not modified by prolonged RBC storage or leukoreduction.28 Additional studies from the same group of investigators showed that RBC supernatant potentiated the secretion of pro-inflammatory cytokines from peripheral blood mononuclear cells exposed to lipopolysaccharide.29
During storage bioactive lipids and other soluble biologic mediators accumulate in the blood component and might be responsible for adverse transfusion reactions in the recipient. RBCs present in either non-leukodepleted or leukodepleted blood units undergo hemolysis in a time-dependent manner during storage delivering a burden of iron as part of the RBC storage lesion. Experiments from a murine RBC transfusion model showed that transfusion of older stored leukoreduced RBCs, but not fresh RBCs, induced a pro-inflammatory state associated with an increased plasma non-transferrin bound iron and linked pro-oxidant effects of increased organ tissue iron.30 The potential post-transfusion inflammatory effect associated with the increased plasmatic iron derived from clearance of transfused older, stored RBCs could explain the increased risk of bacterial infections, multiorgan failure and increased mortality reported by some clinical studies.3,31
The level of pro-inflammatory cytokines (IL-1β, IL-8, tumor necrosis factor-α and monocyte chemoattractant protein) and some endothelium immunoactivation markers (plasma macrophage inhibitory factor and soluble intracellular adhesion molecule-1) have recently been shown to be increased 2–4h after transfusion of RBCs in preterm infants.32 The levels of cytokines have also been analyzed in response to trauma and transfusion. Following human or murine traumatic injury there was an early pro and anti-inflammatory response characterized by elevations of IL-6, IL-10, matrix metalloproteinase-9 (MMP-9), and IFN-γ, while transfusion caused rise in MCP-1, IL-1α, IL-5, IL-15, and sE-selectin levels.33
Over the past two decades the potential clinical impacts of TRIM have been evaluated in both animal and human studies relating blood transfusion to deleterious inflammatory response, immunity misbalance, postoperative infections, tumorigenesis, and metastasis to possibly attribute a definite causal relationship.3,4
Prognosis of colorectal cancer and blood transfusionAfter being on the scene for decades it seems that the issue of prognosis of colorectal cancer and ABT came to long paucity. Various studies have been published relating ABT and long-time survival in colorectal cancer. Although some of these studies did not find a positive correlation,34,35 many others showed that perioperative ABT may decrease 5-years survival of patients who undergone a curative resection of a colorectal carcinoma.36–39 In this context, we should have in mind that the circumstances under which patients are receiving ABT perioperatively are expected to influence cancer recurrence and prognostic. Pre-storage leukoreduction of red cell units is now becoming a routine based on the benefits of reducing post-transfusion infections, preventing febrile transfusion reactions, and decreasing the likelihood of HLA alloimmunization and platelet refractoriness in the recipient.4 In addition, new protocols and guidelines of perioperative management of non-cardiac operations are consistently limiting the prescription of ABT.40,41 The correct identification and implementation of best transfusion practices on the basis of evidence-based clinical trials, published clinical practice guidelines, and process improvements for blood use and clinical patient outcomes have been updated toward a more restrict use of ABT in non-cardiac surgery cases.40–42 Traditionally perioperative anemia was corrected with ABT in a prophylactically fashion when the concentration of hemoglobin was around or less than 10g/dL.42 A randomized trial however, showed that a low threshold of 8g/dL of hemoglobin did not adversely affect patient outcomes.43 There has been a significant increase in systematic reviews and meta-analyses synthesizing the results of trials comparing low versus high hemoglobin thresholds. A Cochrane systematic review of prospective randomized trials compared high versus low hemoglobin concentration thresholds of 19 trials. The authors concluded that low hemoglobin thresholds were well tolerated and safe.44
A retrospective review of The American College of Surgeons National Surgical Quality Improvement Program from 2005 to 2010 was done for colorectal cancer patients operated on with or without ABT. A total of 3815 (14.07%) out of 27,120 cases had ABTs. Transfusions were associated with increased mortality, morbidity, and length of stay. In addition, patients receiving ≥3 blood units had all the above endpoints significantly greater when compared with those receiving 1–2 blood units. Although the study was done in a retrospective fashion it triggers the possibility of ABT and amount of ABT worsen the postoperative prognosis.45
In fact, some retrospective studies from the 1980’ and early 1990’ have consistently shown that an association of ABT and poor survival in colorectal cancer may exit. Busch et al., in 1993, reported a trial, which included 423 patients undergoing curative resection for colorectal cancer who were randomized to receive ABT (n=216) or autologous blood transfusion (n=207) if necessary. However, 164 patients did not receive any type of transfusion (103 in the ABT group and 61 in the autologous group). There was no difference between the two groups on the survival but the disease-free survival in the non-transfused patients was greater than in patients who received any type of transfusion.39
Froman et al. have recently reported the results of a transfusion reducing initiative (TRI) in a teaching hospital. A total of 368 patients were included in this 2-period study: 272 patients in the pre-TRI group and 96 in the post-TRI group. Transfusion rates decreased in the post-TRI group compared with the pre-TRI group (15% vs 28%, P=0.01). The 30-days mortality was similar but the 4-years survival rate of patients with stages I to III adenocarcinoma was greater in non-transfused patients than in transfused patients.46 However, not all studies agree on that. However, one large Swedish study has not found association between red cell transfusion and incidence of disease recurrence and survival in colorectal cancer.47
Improvements in the surgical technique have decreased the amount of intraoperative blood loss occurring during colorectal resections. The volume of blood loss during operation may be greater in open than in videolaparoscopic colonic resection. A meta-analysis with 15 randomized trials and 6557 colorectal cancer cases showed that when operations were performed by laparoscopy the volume of blood loss was significantly less when compared to open operations.48 A decreased long-term survival was observed in patients with colorectal cancer treated with laparoscopic resection however the data analysis suggested that the association between ABT and short survival reflected the poorer medical condition of patient submitted to surgery rather than a causative relationship.36
Finally, a recent systematic review and meta-analysis including 36 clinical observational studies, with a total of 174,036 patients has pointed out the negative effects of perioperative blood transfusion in colorectal cancer surgery. Perioperative transfusion not only decreased overall survival but also reduced and cancer-specific survival. Furthermore, this meta-analysis also showed that perioperative transfusion could increase infectious complications (RR, 1.89, 95% CI, 1.56–2.28; P<0.0001), pulmonary complications (RR, 2.01; 95% CI, 1.54–2.63; P<0.0001), cardiac complications (RR, 2.20; 95% CI, 1.75–2.76; P<0.0001), anastomotic complications (RR, 1.51; 95% CI, 1.29–1.79; P<0.0001), reoperation (RR, 2.88; 95% CI, 2.05–4.05; P<0.0001), and general complications (RR, 1.86; 95% CI, 1.66–2.07; P<0.0001) in colorectal cancer patients undergoing surgert.49 Taking all into account, the overall data suggest that perioperative transfusion causes a dramatically negative effect on long-term prognosis and increases the short-term complications after colorectal cancer surgery.
An important issue that may be associated with the deleterious effects of blood transfusion is the time of storage of the blood though storage may be optimized by adding nutrients, phosphate, and adenine.50 Oxidative damage to the red cell membrane may occur during storage due to depletion of 2, 3-DPG and ATP, and membrane phospholipid vesiculation. All these factors may contribute to corpuscular changes in the RBC during storage.4 The duration of storage of transfused RBCs has been associated with poor clinical outcomes in some studies. However, in one study, patients undergoing resection for colonic cancer and receiving ABT stored for 21 days or more had a longer survival than others receiving ABT stored less than 21 days. Best prognosis however was associated with non-transfused cases: the median overall survival was 4.6 years for 288 non-transfused patients and 3.0 years for 452 transfused patients.51 A recent study in a heterogeneous population of patients with cancer was not able to associated transfusion of units stored for more or less than 14 days with long-time survival.52
Final remarks and conclusionsAlthough it has been well demonstrated that TRIM is associated with immune alterations by qualitative and quantitative laboratory tests its influence in colorectal cancer recurrence after a curative resection remains controversial though probably may exist. New surgical techniques reducing intraoperative blood loss and recent blood transfusions restrictive studies have limited the number of ABTs. However, increase in mortality continues to be reported in literature after perioperative transfusion in colorectal cancer surgery.49,53 Poor survival in colorectal cancer is probably associated with the total volume of ABT, and the length of blood storage.
Conflicts of interestThe authors declare no conflicts of interest.