Hemolysis due to ABO incompatibility is an important differential diagnosis in newborns presenting with jaundice. Clinical studies evaluating ABO hemolytic disease of fetus and newborn (ABO-HDFN) question the diagnostic value of the direct antiglobulin test (DAT) in this situation.
GoalsTo determine the clinical and laboratorial findings associated with the occurrence of ABO-HDFN and to evaluate the accuracy of DAT as a diagnostic tool.
MethodsThis was a nested case control study with a cohort of 4122 newborns. Clinical and immunohematological data were retrieved from medical files including clinical and laboratorial factors associated with ABO-HDFN. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of positive DAT were calculated.
ResultsAmong the 4122 newborns, 44 had the diagnosis of ABO-HDFN. Positive DAT, group O mother and group A newborn were significantly associated with the occurrence of neonatal jaundice and this association persisted in a multivariable model (p-value <0.001). DAT presented 65.85 % sensitivity, 96.28 % specificity, 16.9 % PPV and 99.6 % NPV for the diagnosis of ABO-HDFN. There were no cases of positive DAT in cases other than O/A and O/B incompatibilities. The newborn hemoglobin was significantly lower in O/A incompatibility (p-value <0.001).
ConclusionPositive DAT, mother of group O and newborn of group A are independent risk factors associated with ABO-HDFN. DAT exhibited high NPV for the diagnosis of this complication. Thus, performing DAT in newborns with O/A and O/B incompatibilities is a cost-effective strategy that can be applied as routine by blood banks.
Hemolytic disease of the newborn (HDNB) due to ABO incompatibility is a common cause of neonatal hyperbilirubinemia and jaundice; usually it presents with a mild course. The physiopathology of the disease relies on the presence of anti-A and anti-B antibodies, mostly of the IgG2 class, that cross the placenta barrier and sensitize fetal red blood cells (RBCs) which still do not exhibit full expression of A and B antigens.1 The scenario of mother and fetus O/A and O/B incompatibilities exhibit the highest risk of neonatal jaundice.2
The routine of immunohematological tests on newborns at birth is not homogeneous in blood centers. Blood banks usually perform direct antiglobulin test (DAT) for all newborns, irrespective of the ABO group of the mother. Some commercial assays designed for ABO typing of newborns under automation even link anti-A and anti-B sera with anti-human globulin to perform polyspecific DAT. This is the case of gel-method tests designed for newborns, in which one microcolumn of agglutination is reserved for anti-A, one for anti-B and one for anti-human globulin. A survey conducted by the AABB Pediatric Subsection Working Party among US and Canadian birth hospitals confirmed the heterogeneity in the newborn immunohematology routine, mainly in respect to the performance of DAT (mandatory versus optional) and eluate.3 However, studies focusing on the clinical management of neonatal jaundice show that the diagnosis of ABO-HDFN should focus mostly on the clinical course of hyperbilirubinemia in the context of newborns with ABO incompatible mothers than on positive DAT.4
Our main objectives were to evaluate clinical and laboratory findings associated with the occurrence of neonatal jaundice due to mother and fetus ABO incompatibility and to calculate the positive and negative predictive value of DAT in the diagnosis of this complication.
MethodsDescription of the cohortMother and newborns were included in the study sequentially as post-delivery samples were tested in three Brazilian immunohematology laboratories. A total of 4122 full-term newborns were included in the study. Hemolytic disease of the newborn (HDNB) was suspected in the presence of overt jaundice with confirmed increased indirect bilirubin. Clinical and laboratorial data were retrieved from hospital files.
Immunohematological testsAll newborn and mother samples were ABO typed using the tube method with commercial anti-A and anti-B sera (BIORAD, Lagoa Santa, Brazil). Only direct ABO typing was performed after washing cord red blood cells (RBCs) eight times with room temperature saline. All newborn samples underwent polyspecific DAT using the tube method with commercial anti-human globulin (Fresenius Kabi, Brazil).
Statistical analysisNewborns presenting with ABO-HDFN were compared to control newborns in respect to clinical and laboratorial variables. The chi-square test was used to compare the groups in univariate analysis and if the resulting p-value <0.2, variables were included in the multivariable model. Logistic regression was performed for multivariable analysis and a p-value <0.05 was considered significant.
The frequency of positive DAT and the neonatal hemoglobin were compared between groups of newborns with different ABO blood types stratified according to the ABO group of the mother. The chi-square test was used for the first comparison and Mann–Whitney test for the second. For both, a p-value <0.05 was considered significant. All tests were performed using SPSS (version 20).
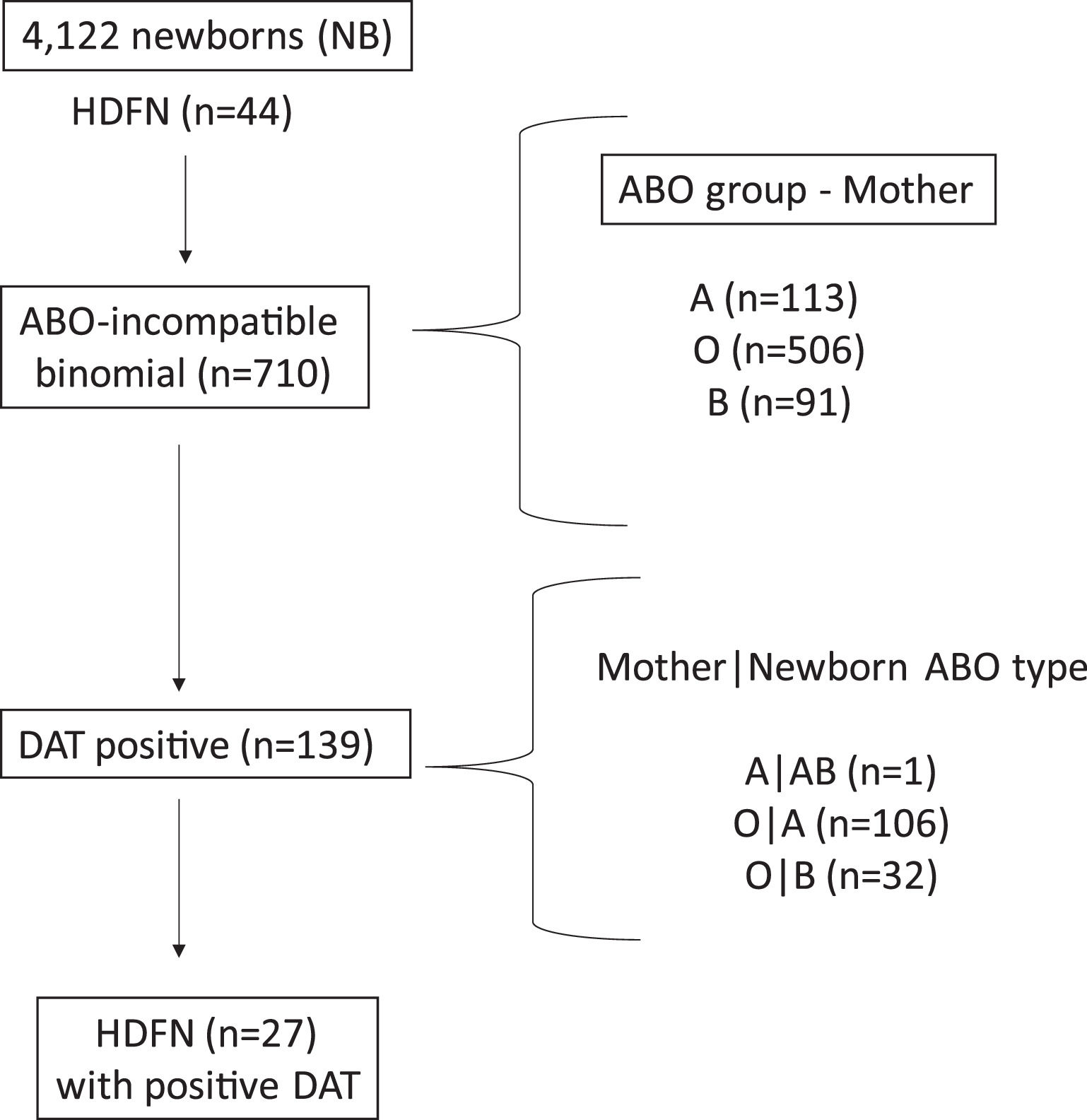
ResultsCohort descriptionOf the 4122 newborns enrolled in the study, 44 had the diagnosis of ABO-HDFN. The incidence of this complication was 7.9 % considering only cases in which there was ABO incompatibility between mother and fetus. Figure 1 shows a summary of the immunohematological results.
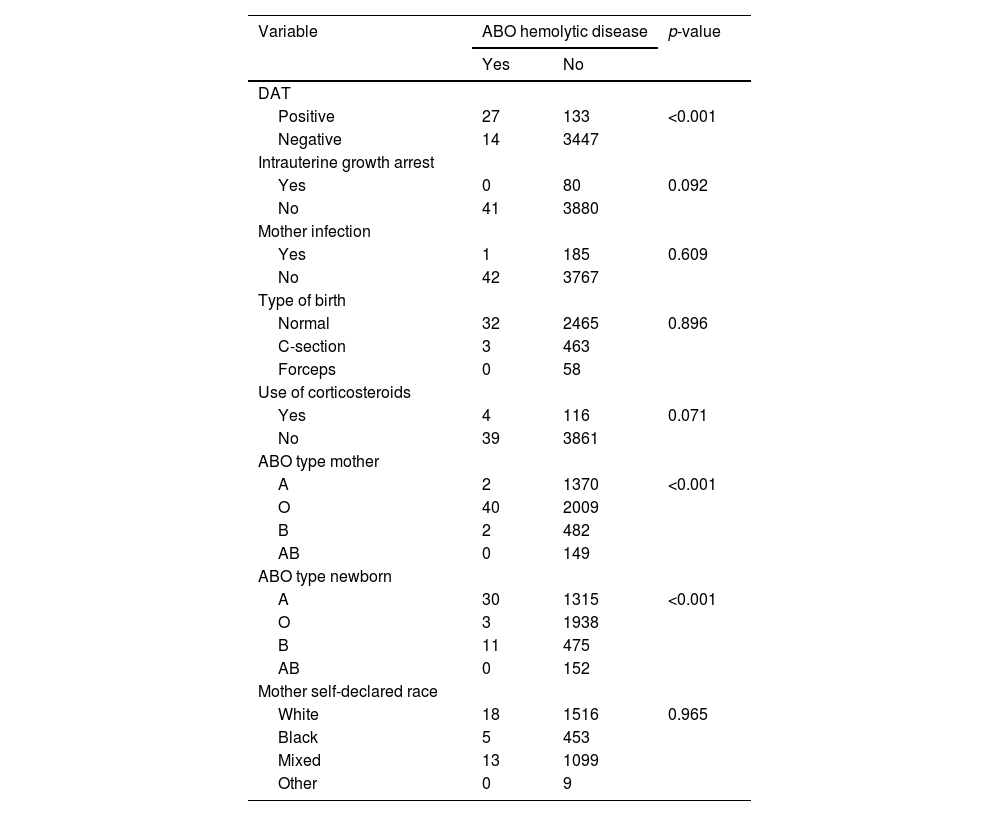
Clinical and laboratorial factors associated with newborn jaundicePositive DAT, and ABO group of the mother and of the newborn were significantly associated with clinical neonatal jaundice (p-value <0.001) in univariate analysis (Table 1). Intrauterine growth arrest, bacterial infection of the mother, mother's self-declared race and type of birth were not related to the studied endpoint (p-value >0.2; Table 1). Maternal use of corticosteroids was included in the multivariable model, as the p-value was below 0.071. In the multivariable analysis, only positive DAT, and ABO group of the mother and of the newborn exhibited independent associations with newborn jaundice (p-value <0.01).
Univariate analysis of clinical and laboratorial factors potentially associated with ABO-HDFN.
The sensitivity, specificity, positive predictive value and negative predictive value of positive DAT for the diagnosis of neonatal jaundice were 65.85 %, 96.28 %, 16.88 % and 99.6 %, respectively.
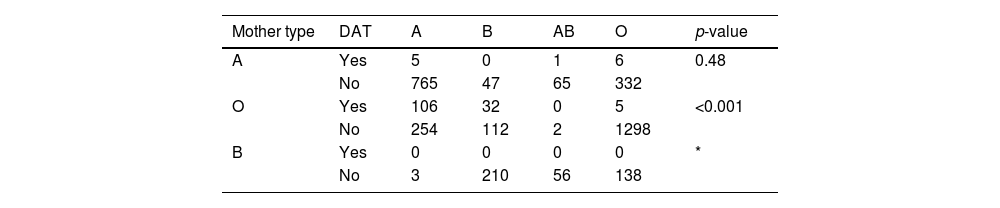
Direct antiglobulin test (DAT)DAT was positive in 19.6 % of the mother and newborn pairs with ABO incompatibility (139/710). Considering the ABO group of the mother, DAT was positive in 0.9 % of newborns of group A mothers (1 ABO incompatible newborn in 113 births), 27.3 % of newborns of group O mothers (138 ABO incompatible newborns in 506 births) and in no newborns of group B mothers (no ABO incompatible newborns in 91 births). The higher prevalence of positive DAT in mothers of group O presenting with type A or B newborns was statistically significant (p-value <0.001; Table 2). The frequency of positive DAT for newborns of group O mothers was 29.4 % in the case of group A newborns and 22.2 % in the case of group B newborns. There were 16 newborns presenting with positive DAT in the absence of ABO incompatibility. The eluate, in these cases, did not reveal anti-A or anti-B antibodies.
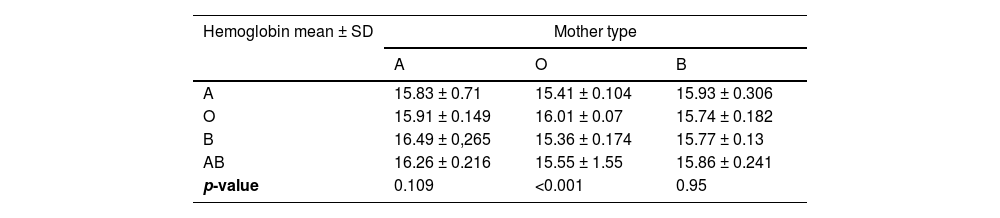
Impact of ABO incompatibility on the newborn hemoglobinA significant decrease in post-delivery newborn hemoglobin was observed in newborns of group A with group O mothers (p-value <0.001; Table 3). ABO incompatibility did not significantly impact the hemoglobin of ABO-incompatible newborns of group A or group B mothers (p-value = 0.109 and 0.95, respectively).
DiscussionThis nested case-control study evaluated a large cohort of mother and newborn pairs focusing on immunohematological findings associated with the occurrence of neonatal jaundice due to ABO incompatibility. It was observed that: 1) Positive DAT, mother of group O and newborn of group A were variables significantly and independently associated with neonatal jaundice, 2) Newborn hemoglobin levels were lower in O/A incompatibility. In cases of non-group O mothers, no differences were observed in neonatal hemoglobin irrespective of ABO incompatibility and 3) The frequency of positive DAT was significantly higher in O/A and O/B incompatibilities. In the scenario of group B mothers, no DAT was positive, including in cases where newborns were of type A.
This study is the first to explore major ABO incompatibility involving mother and newborn pairs in Brazil, a medium-income country of highly mixed population. In most Brazilian centers the routine tests performed at birth for all newborns include DAT, with eluate in cases of positive results, and, in some centers, eluate is performed in cases of O/A and O/B incompatibility irrespective of DAT result.
The results of this study show a positive DAT frequency of 19.6 % in the presence of ABO incompatibility between mother and newborn. This result is in accordance with previously published data from different populations. The highest frequency of positive DAT found in O/A and O/B incompatibility was also reported by other studies, reinforcing the evidence that group O mothers have greater chances of having babies with positive DAT.1,2,5
The calculated sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of positive DAT for the diagnosis of neonatal jaundice in this study were 65.85 %, 96.28 %, 16.88 % and 99.6 %, respectively. The poor PPV of DAT in the scenario of ABO-HDFN has been reported by other studies, as has the high NPV.2,6
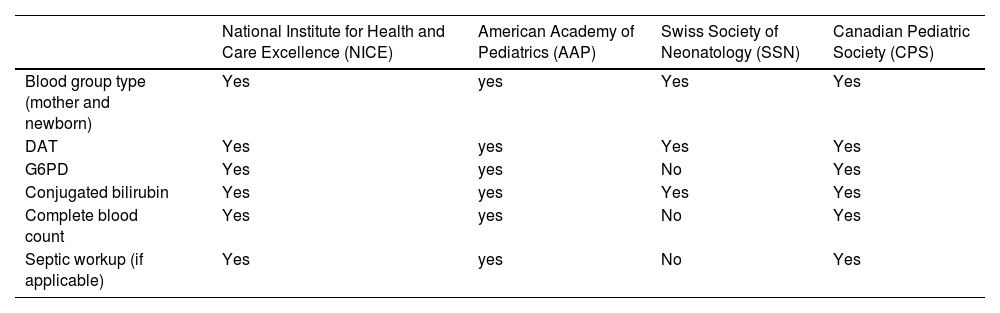
There is a great discussion concerning the role played by DAT in the diagnosis and prognosis of ABO-HDFN. Studies focusing on the clinical aspects of ABO-HDFN point out that a positive DAT is not a very helpful diagnostic tool because of the low PPV. This means that presenting positive DAT will not confirm the diagnosis of HDFN. This is very important information to highlight as neonatal jaundice has multiple differential diagnoses (red blood cell membrane and enzyme disorders, physiological jaundice, liver diseases, among others). Even though most guidelines recommend performing DAT to investigate prolonged neonatal jaundice, as shown in Table 4, the interpretation of the results should take into consideration the clinical course of the disease and the exclusion of other possible causes in more severe cases (glucose-6-phosphate dehydrogenase deficiency and sepsis, for example).3
Laboratorial tests indicated for the evaluation of prolonged neonatal jaundice.
DAT, direct antiglobulin test; G6PD, glucose-6-phosphate dehydrogenase deficiency.
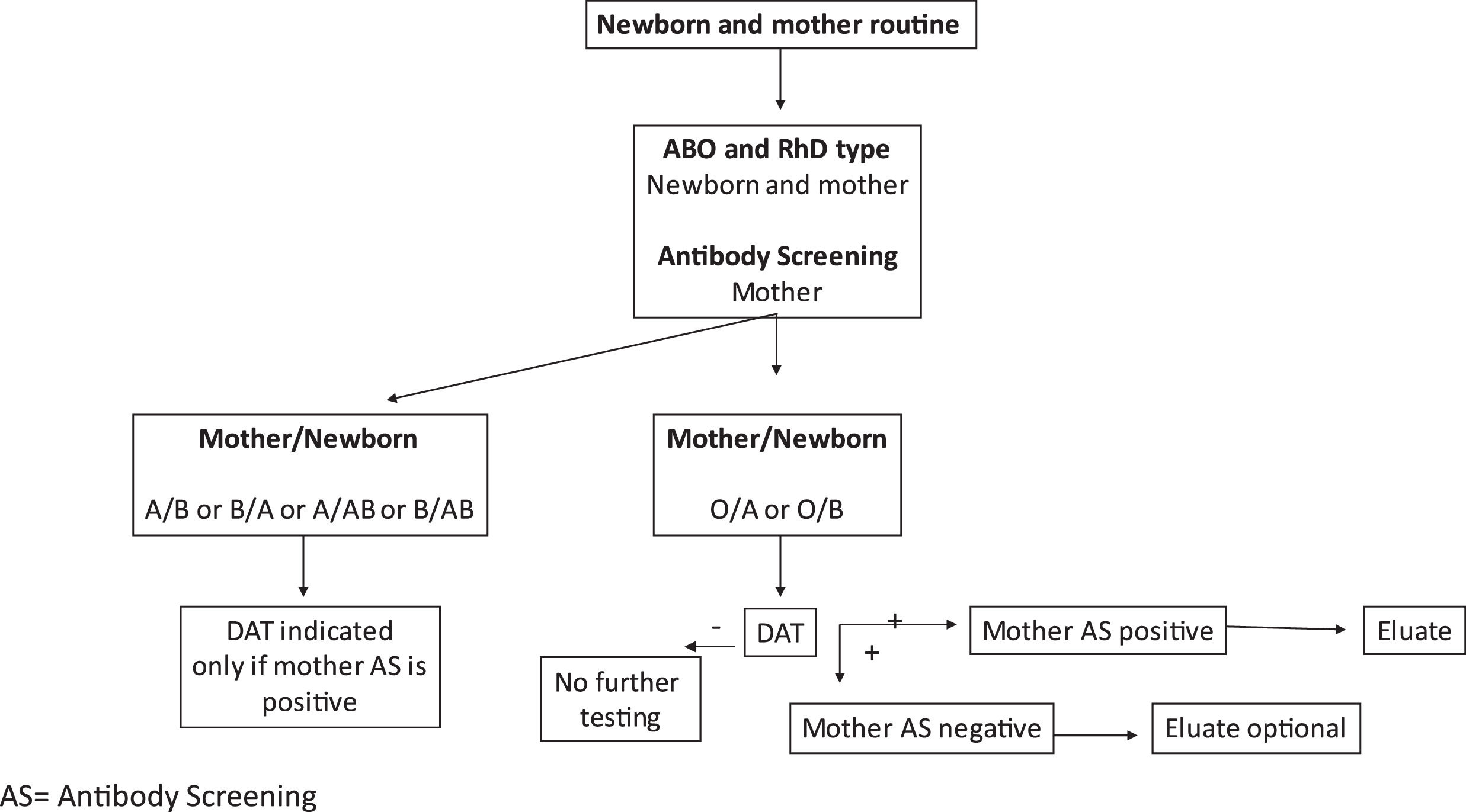
In the immunohematology laboratories, the high NPV of DAT sheds light on the need to re-evaluate the newborn routine, as the practice of performing eluate to identify small amounts of anti-A or anti-B in cases of negative DAT seems questionable. Additionally, considering the present results, even DAT could be performed only in cases of O/A and O/B incompatibilities. A suggested guideline for immunohematology laboratories is shown in Figure 2.
ABO-HDFN has a benign course in most cases. However, it is known that for milder cases there is a wide variation in the prevalence of ABO-HDFN, mainly depending on the clinical parameters used to define the cases. Considering the low PPV, defining ABO-HDFN based only on DAT would certainly increase the number of cases diagnosed with this complication. Thus, the diagnosis of ABO-HDFN should be based on clinical suspicion (presence of jaundice correlated with the number of days after delivery) as well as bilirubin measurements.
This study has some limitations. The first refers to the fact that newborn bilirubin levels were not assessed and, as a consequence, the diagnosis of clinical jaundice relied on its description in medical files. The measurement of bilirubin levels in newborns with jaundice was performed by plasma measurement, but the results were not included in the medical files for most patients. The second limitation is that the treatment of newborns with ABO-HDFN was not included in the analysis.
In conclusion, positive DAT and mother-fetus O/A and O/B incompatibilities are risk factors for ABO-HDFN. DAT presents a low PPV for the diagnosis of the disease, but a very high NPV. As such, performing DAT in situations of O/A and O/B incompatibilities may represent the most cost-effective strategy to aid physicians in the differential diagnosis of neonatal jaundice.
FundingNo funding to declare.