Chronic immune thrombocytopenia (cITP) is characterized by dysregulation of the immune response. Until recently, the role of Th2-related cytokine gene polymorphisms was unclear. Interleukin 4 (IL-4) exerts its functions by binding to three types of IL-4 receptor (IL-4R) complexes. We aimed to explore the potential association between the gene polymorphism of IL-4Rα and cITP.
MethodsWe investigated the clinical impact of the IL-4Rα (rs1801275) A>G single nucleotide polymorphism (SNP) using the polymerase chain reaction (PCR) followed by the restriction fragment length polymorphism (RFLP) method in 82 cITP patients and 60 healthy controls (HCs).
ResultsThe IL-4Rα (rs1801275) A>G polymorphism analysis showed the mutant GG genotype was significantly higher in control females (p = 0.033). The wild AA genotype had a higher bleeding score (p = 0.02) in the adulthood onset group. Furthermore, the wild AA genotype in the cITP childhood onset group was significantly associated with the disease severity, as well as the response to treatment (p = 0.040).
ConclusionThe mutant G allele is protective against the susceptibility to cITP in the Egyptian females. The IL-4Rα (rs1801275) A>G polymorphism may affect the clinical severity of cITP and treatment response in the Egyptian population.
Chronic immune thrombocytopenia (cITP) is an autoimmune disorder defined as isolated thrombocytopenia (platelet count < 100 × 103/dL) that lasts for more than a year.1 Increased platelet destruction in the reticuloendothelial system and megakaryocyte inhibition or destruction in the bone marrow, which may be caused by antibodies, autoreactive T cells, or both, are the immune pathogenesis of cITP.2
Numerous studies show that inflammatory cytokines made by Th1 cells are involved in the development of ITP, but until recently, the function of gene polymorphisms related to Th2 cytokines was unclear.3
The pleiotropic cytokine interleukin 4 (IL-4) plays a number of important roles, including the polarization of the T-helper (Th) cell differentiation toward Th2 cells, which in turn suppresses the differentiation of Th1 cells and, as a result, the type 1 inflammation.4 Th1/Th2 balance is crucial for the immune system hemostasis in a physiological state and autoimmune diseases have been linked to disruptions of this balance.5
To exert its function, the IL-4 binds to three types of IL-4 receptor (IL-4R) complexes. The c-common (C) chain of the IL-2R and the IL-4R are the two subunits that compose the type I IL-4R. The IL-4R and the IL-13R are the two subunits that compose the type II IL-4R. The three chains are all present in the type III IL-4R. The IL-4R is, therefore, the essential part of all three complexes. Depending on the type of cell, the IL-4 recruits one of the other two chains in the complexes when it binds to the IL-4R, creating a heterodimer complex that initiates the signal transduction.6
The susceptibility to autoimmune or inflammatory diseases, such as rheumatoid arthritis, systemic lupus erythematosus, Graves' disease, myasthenia gravis, eczema and asthma, as well as the severity and treatment effects of some of the diseases, have all been linked to polymorphisms of the IL-4R gene.6–11
The Arg576Gln polymorphism on the IL-4Rα gene was rarely reported to be associated with the susceptibility to the cITP.3 In the current study, we aimed to explore the potential association between the gene polymorphism of the IL-4Rα and the susceptibility and severity, as well as the response to therapy, in a cohort of Egyptian cITP patients.
Patients and methodsPatientsWe conducted a single-institution case-control study enrolling a total of 82 Egyptian chronic ITP patients; all were recruited from the clinical hematology unit, Internal Medicine Department of the Kasr Al-Ainy teaching hospital, where they were diagnosed and followed up prospectively between February 2020 and September 2021, their diagnoses having been made according to the criteria of the ITP International Working Group (IWG).12 Patients with disorders that may be associated with 2ry thrombocytopenia were excluded from the study. Sixty age and sex-matched unrelated Egyptian individuals living in the same geographical region were enrolled as healthy controls (HCs). Informed consent was obtained from all patients before the study initiation and patient recruitment and the study was approved by the local ethical committee. The study complied with good clinical practice protocols and with the ethical rules stated in the Declaration of Helsinki (as revised in Tokyo in 2004).
MethodsAll subjects with immune thrombocytopenia, as well as healthy controls, were subjected to a full history investigation (especially bleeding, drug intake, family history and compliance to therapy) by means of a clinical examination (especially for skin, organomegaly and lymph nodes) and laboratory investigations, which included the complete blood count (CBC) & film, reticulocyte count and erythrocyte sedimentation rate (ESR). Patients with secondary ITP due to viral infections (hepatitis B, hepatitis C and human immunodeficiency viruses), drug-induced afflictions, H. Pylori and autoimmune diseases, such as SLE, and known thyroid disease were excluded.
The cITP was defined as an isolated thrombocytopenia (platelet count < 100 × 103/mm3) which persisted for over 12 months.
Definitions of response:
- •
Complete response: platelet count ≥100 × 103/mm3 and absence of bleeding.
- •
Response: platelet count ≥30 × 103/mm3 and at least a 2-fold increase in the baseline count and absence of bleeding.
- •
No response: platelet count <30 × 103/mm3 or less than a 2-fold increase of baseline platelet count or bleeding.13
Corticosteroid dependence was defined as the continuous need for corticosteroid therapy to preserve a platelet count at or above 30 × 103/mm3 and/or to avoid bleeding. The ITP bleeding scale (IBLS) was used as an assessment system for the severity of bleeding, with grades from 0 (none) to 2 (marked bleeding), according to the Page et al. protocol.14
GenotypingWhole blood collected in ethylenediaminetetraacetic acid (EDTA) was subjected to genomic DNA extraction using the DNA extraction kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer's protocol. The IL-4Rα A>G (rs1801275) genotypes were determined using the polymerase chain reaction (PCR) followed by the restriction fragment length polymorphism (RFLP) technique. The Sequence of the primers used for amplification was: 5′-GCCCCCACCAGTGGCTACC-3′ (forward primer) and 5′-GAGGTCTTGGAAAGGCTTATAC-3′ (Reverse primer) (Invitrogen, Thermo Scientific, USA). A total PCR reaction mixture of 25 μL containing 4 μL of purified genomic DNA, 1 μL of each forward and reverse primers, 12.5 μL Reaction Mix and 6.25 μL ddH2O. The cycling conditions of the PCR were as follows: denaturation at 95 °C for 4 min., followed by 30 cycles of 94 °C for 30 s., then annealing at 59.5 °C for 30 s and 72 °C for 30 s. A final extension step at 72 °C for a 7-min. digestion of PCR products with restriction enzyme (MspI) (Thermo Scientific, Waltham, MA, USA) was performed according to the manufacturer's protocol. The PCR products were separated on agarose gel, visualized by ethidium bromide stain and ultraviolet light transillumination. The digested products yielded a 204 bp fragment which represented the GG genotype, 184 bp and 18 bp fragments, representing the AA genotype, and 204, 184 and 18 bp, representing the AG genotype. Twenty percent of the samples were repeated twice randomly for confirmation and the results were 100% the same.
Statistical analysisData were coded and entered using the statistical package SPSS (Statistical Package for the Social Sciences), version 25. Data were summarized using median, minimum and maximum in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were performed using the non-parametric Mann-Whitney test. For comparing categorical data, the chi-square (χ2) test was performed or, instead, the exact test, when the expected frequency was less than 5. The genotype and allele frequencies were compared between the different groups using chi-square tests. The odds ratio (OR) with 95% confidence intervals was calculated. The p-values less than 0.05 were considered statistically significant.
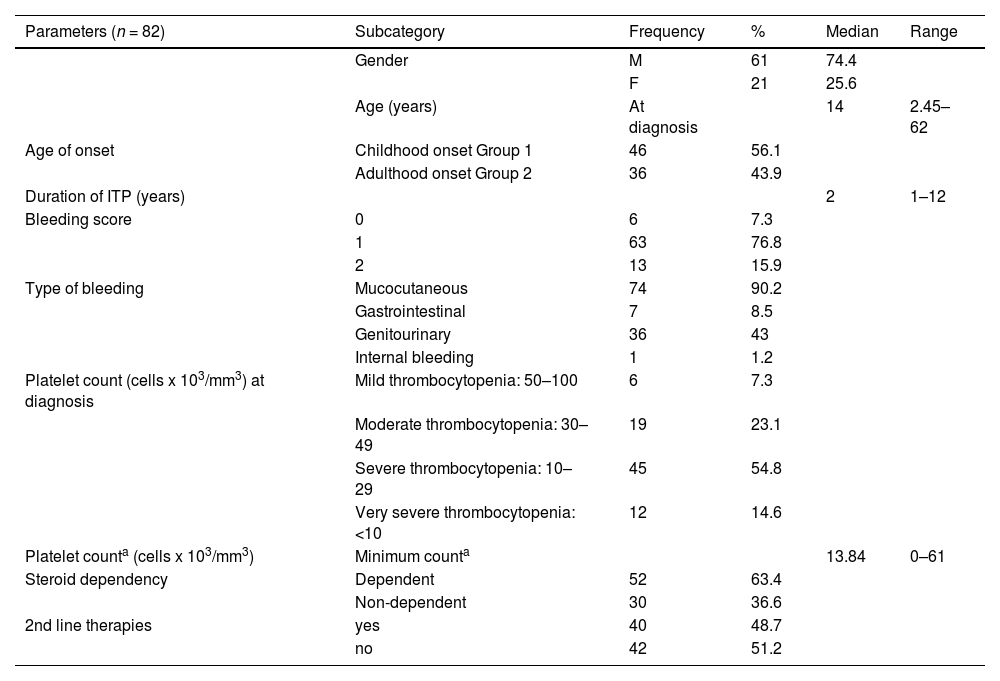
ResultsIn the present case-control study, the median age of cITP patients at sampling was 29.5 years, their median age at diagnosis was 14 years and the median age of the healthy control group was 31.2 years. The female gender constituted 74.4% of patients, while 25.6% were males. Clinical and demographic characteristics of cITP patients are summarized in Table 1.
Parametric and non-Parametric data of studied cITP patients.
| Parameters (n = 82) | Subcategory | Frequency | % | Median | Range |
|---|---|---|---|---|---|
| Gender | M | 61 | 74.4 | ||
| F | 21 | 25.6 | |||
| Age (years) | At diagnosis | 14 | 2.45–62 | ||
| Age of onset | Childhood onset Group 1 | 46 | 56.1 | ||
| Adulthood onset Group 2 | 36 | 43.9 | |||
| Duration of ITP (years) | 2 | 1–12 | |||
| Bleeding score | 0 | 6 | 7.3 | ||
| 1 | 63 | 76.8 | |||
| 2 | 13 | 15.9 | |||
| Type of bleeding | Mucocutaneous | 74 | 90.2 | ||
| Gastrointestinal | 7 | 8.5 | |||
| Genitourinary | 36 | 43 | |||
| Internal bleeding | 1 | 1.2 | |||
| Platelet count (cells x 103/mm3) at diagnosis | Mild thrombocytopenia: 50–100 | 6 | 7.3 | ||
| Moderate thrombocytopenia: 30–49 | 19 | 23.1 | |||
| Severe thrombocytopenia: 10–29 | 45 | 54.8 | |||
| Very severe thrombocytopenia: <10 | 12 | 14.6 | |||
| Platelet counta (cells x 103/mm3) | Minimum counta | 13.84 | 0–61 | ||
| Steroid dependency | Dependent | 52 | 63.4 | ||
| Non-dependent | 30 | 36.6 | |||
| 2nd line therapies | yes | 40 | 48.7 | ||
| no | 42 | 51.2 |
On analysis of the clinical and laboratory data of cITP patients, we found that the bleeding score was 0, 1, and 2 in (7.3%, 76.8%, 15.9% of patients, respectively). The median minimum platelet count was 13.84 × 103/mm3, with a range of 0 to 61 × 103/mm3.
On following up with our cITP patients for 1 year, only 14.6% of patients suffered from very severe ITP, 63% were steroid dependent and 48% received second-line therapies.
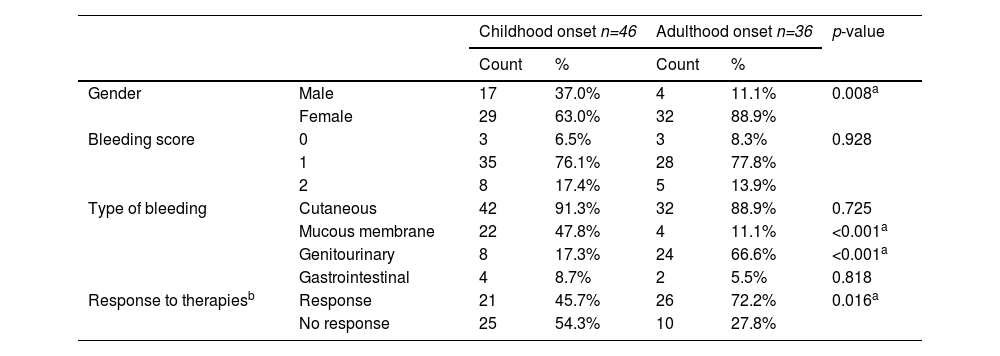
Patients were sub-grouped into 2 subgroups, Group 1 being childhood onset ITP (n= 46) (defined from age 0 to 14 years) and Group 2, adulthood onset ITP (n= 36) (defined from age ≥ 14 years). Clinical and laboratory variations between both groups are shown in Table 2.
Comparison between childhood onset & adulthood onset cITP patients.
| Childhood onset n=46 | Adulthood onset n=36 | p-value | ||||
|---|---|---|---|---|---|---|
| Count | % | Count | % | |||
| Gender | Male | 17 | 37.0% | 4 | 11.1% | 0.008a |
| Female | 29 | 63.0% | 32 | 88.9% | ||
| Bleeding score | 0 | 3 | 6.5% | 3 | 8.3% | 0.928 |
| 1 | 35 | 76.1% | 28 | 77.8% | ||
| 2 | 8 | 17.4% | 5 | 13.9% | ||
| Type of bleeding | Cutaneous | 42 | 91.3% | 32 | 88.9% | 0.725 |
| Mucous membrane | 22 | 47.8% | 4 | 11.1% | <0.001a | |
| Genitourinary | 8 | 17.3% | 24 | 66.6% | <0.001a | |
| Gastrointestinal | 4 | 8.7% | 2 | 5.5% | 0.818 | |
| Response to therapiesb | Response | 21 | 45.7% | 26 | 72.2% | 0.016a |
| No response | 25 | 54.3% | 10 | 27.8% | ||
n: number.
A comparison of clinical characteristics of both subgroups of cITP revealed that the median age of diagnosis in the childhood onset group was 5.79 years, while it was 20.53 years in the adulthood onset group. There was a statistically significant difference in gender between both groups (p = 0.008), females were more affected in the adulthood onset cITP group. The site of bleeding shows a significant difference between both groups, as children suffered more from mucous membrane bleeding, while adults experienced more genitourinary bleeding (p < 0.001), but there was no statistically significant difference between both groups regarding bleeding scores. During the course of therapy, the maximum platelet response showed a significant difference in the childhood onset group in comparison with the adulthood onset group (p = 0.018). Moreover, the adulthood onset group response to therapy was significantly higher than the childhood onset group (p= 0.016).
Genotype frequencies of the IL4R α (A>G) SNP, were in Hardy–Weinberg equilibrium in both (the control and cITP) groups (p = 0.344 and 0.05, respectively).
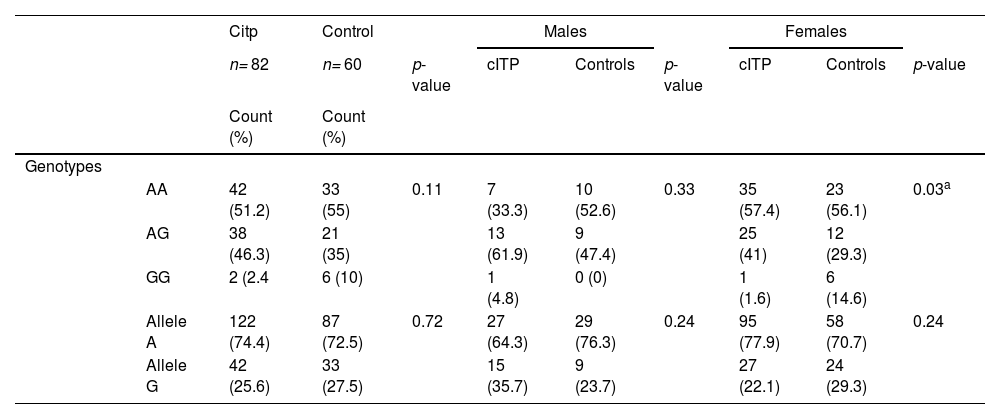
Analysis of the genotype and allele frequencies of the IL-4Rα revealed that the proportion of the wild homozygous (AA) genotype and (A) allele was the highest and that of mutant homozygous (GG) genotype and (G) allele was the lowest in both cITP and control groups.
On analyzing the genotype and allele frequencies in cITP patients in dominant and recessive models, we found that cITP patients had a lower frequency of the IL-4Rα homozygous mutant GG genotype, in comparison with healthy controls (2.4% vs. 10%, respectively, odds ratio (OR) = 0.225), however, this association was not statistically significant (Table 3).
IL-4Rα gene polymorphism in cITP, as compared to controls, as well as gender comparison.
| Citp | Control | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n= 82 | n= 60 | p-value | cITP | Controls | p-value | cITP | Controls | p-value | ||
| Count (%) | Count (%) | |||||||||
| Genotypes | ||||||||||
| AA | 42 (51.2) | 33 (55) | 0.11 | 7 (33.3) | 10 (52.6) | 0.33 | 35 (57.4) | 23 (56.1) | 0.03a | |
| AG | 38 (46.3) | 21 (35) | 13 (61.9) | 9 (47.4) | 25 (41) | 12 (29.3) | ||||
| GG | 2 (2.4 | 6 (10) | 1 (4.8) | 0 (0) | 1 (1.6) | 6 (14.6) | ||||
| Allele A | 122 (74.4) | 87 (72.5) | 0.72 | 27 (64.3) | 29 (76.3) | 0.24 | 95 (77.9) | 58 (70.7) | 0.24 | |
| Allele G | 42 (25.6) | 33 (27.5) | 15 (35.7) | 9 (23.7) | 27 (22.1) | 24 (29.3) | ||||
Furthermore, on stratification of the cITP group according to the age of onset, the frequency of the IL-4Rα homozygous mutant GG genotype was also lower in both adulthood onset and childhood onset groups, in comparison with healthy controls (4.3% and 0%, vs. 10%, respectively), and this association was also not statistically significant.
The cITP group was further stratified by gender and the analysis of the distribution of genotype frequencies revealed that the effect of the homozygous mutant GG genotype is more pronounced in females, as the GG genotype was higher in control females than in female cITP patients (14.6% vs. 1.6%, respectively; OR = 6.968), and this association was statistically significant (p = 0.033) (Table 3).
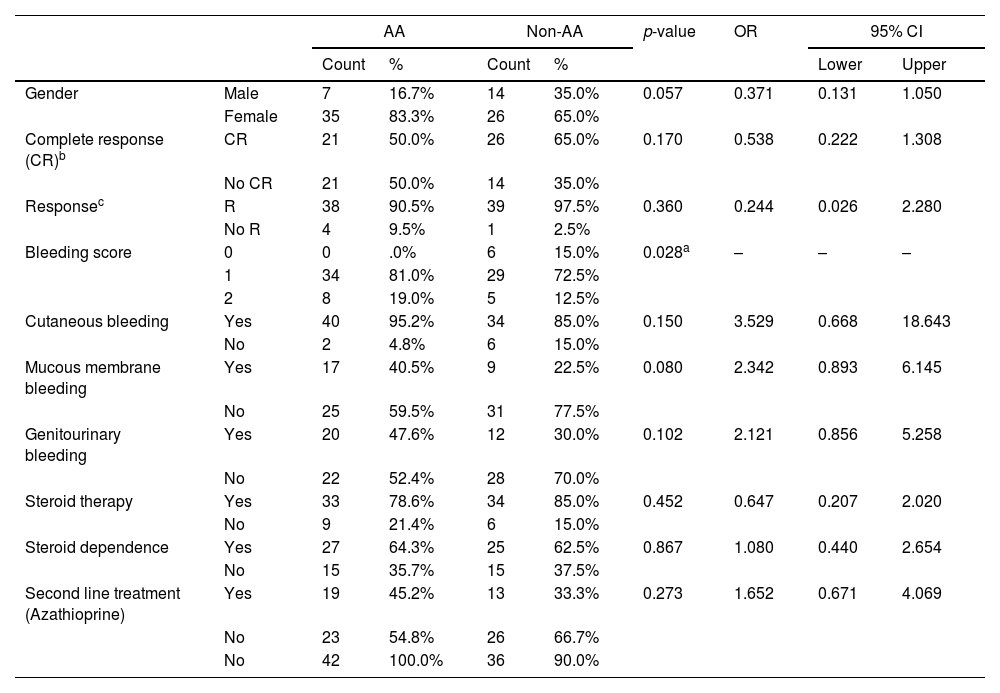
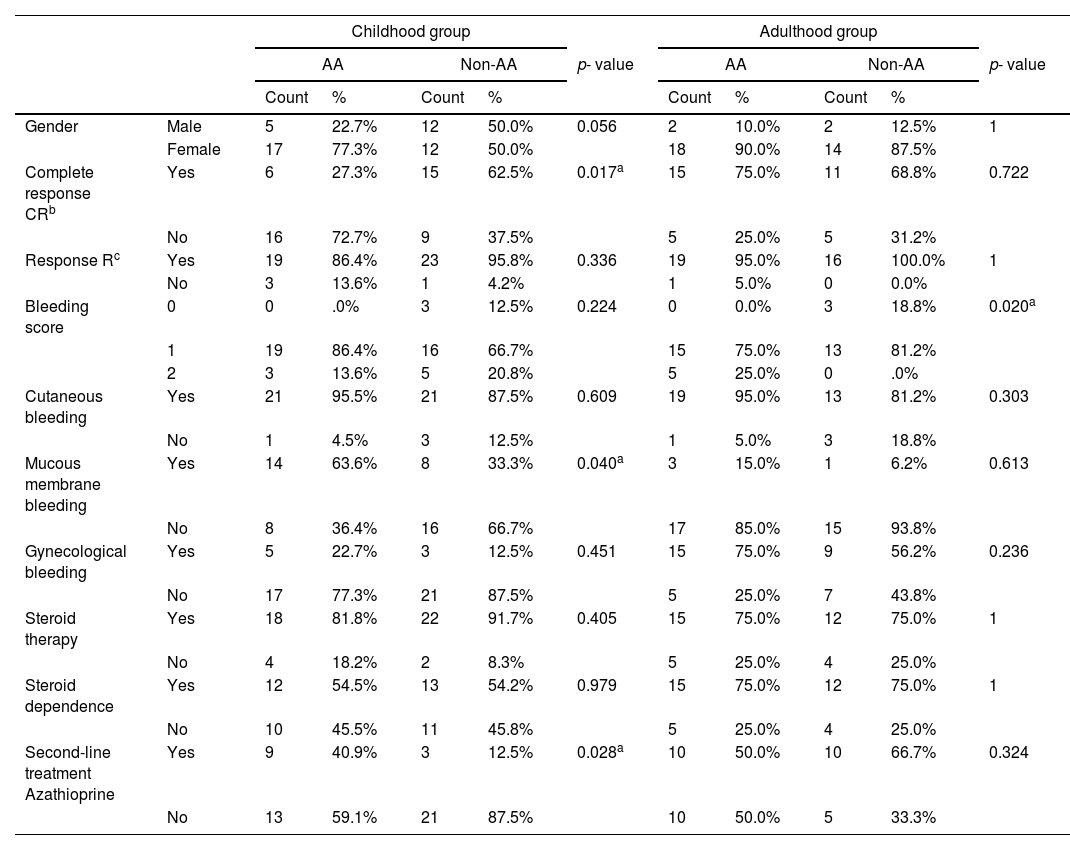
Examination of the association between the IL-4Rα A>G polymorphism and the clinical and demographic characteristics of cITP patients demonstrated that carriers of the AA genotype have higher bleeding scores than non-AA genotype carriers and this association was of statistical significance in cITP, as well as in the adulthood onset group (p = 0.028 and 0.02, respectively), however, it was not of statistical significance in the cITP childhood onset group of (Tables 4 and 5).
Association IL-4Rα genotypes and cITP characteristics.
| AA | Non-AA | p-value | OR | 95% CI | |||||
|---|---|---|---|---|---|---|---|---|---|
| Count | % | Count | % | Lower | Upper | ||||
| Gender | Male | 7 | 16.7% | 14 | 35.0% | 0.057 | 0.371 | 0.131 | 1.050 |
| Female | 35 | 83.3% | 26 | 65.0% | |||||
| Complete response (CR)b | CR | 21 | 50.0% | 26 | 65.0% | 0.170 | 0.538 | 0.222 | 1.308 |
| No CR | 21 | 50.0% | 14 | 35.0% | |||||
| Responsec | R | 38 | 90.5% | 39 | 97.5% | 0.360 | 0.244 | 0.026 | 2.280 |
| No R | 4 | 9.5% | 1 | 2.5% | |||||
| Bleeding score | 0 | 0 | .0% | 6 | 15.0% | 0.028a | – | – | – |
| 1 | 34 | 81.0% | 29 | 72.5% | |||||
| 2 | 8 | 19.0% | 5 | 12.5% | |||||
| Cutaneous bleeding | Yes | 40 | 95.2% | 34 | 85.0% | 0.150 | 3.529 | 0.668 | 18.643 |
| No | 2 | 4.8% | 6 | 15.0% | |||||
| Mucous membrane bleeding | Yes | 17 | 40.5% | 9 | 22.5% | 0.080 | 2.342 | 0.893 | 6.145 |
| No | 25 | 59.5% | 31 | 77.5% | |||||
| Genitourinary bleeding | Yes | 20 | 47.6% | 12 | 30.0% | 0.102 | 2.121 | 0.856 | 5.258 |
| No | 22 | 52.4% | 28 | 70.0% | |||||
| Steroid therapy | Yes | 33 | 78.6% | 34 | 85.0% | 0.452 | 0.647 | 0.207 | 2.020 |
| No | 9 | 21.4% | 6 | 15.0% | |||||
| Steroid dependence | Yes | 27 | 64.3% | 25 | 62.5% | 0.867 | 1.080 | 0.440 | 2.654 |
| No | 15 | 35.7% | 15 | 37.5% | |||||
| Second line treatment (Azathioprine) | Yes | 19 | 45.2% | 13 | 33.3% | 0.273 | 1.652 | 0.671 | 4.069 |
| No | 23 | 54.8% | 26 | 66.7% | |||||
| No | 42 | 100.0% | 36 | 90.0% | |||||
Association IL-4Rα genotypes and ITP characteristics in both groups.
| Childhood group | Adulthood group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | Non-AA | p- value | AA | Non-AA | p- value | ||||||
| Count | % | Count | % | Count | % | Count | % | ||||
| Gender | Male | 5 | 22.7% | 12 | 50.0% | 0.056 | 2 | 10.0% | 2 | 12.5% | 1 |
| Female | 17 | 77.3% | 12 | 50.0% | 18 | 90.0% | 14 | 87.5% | |||
| Complete response CRb | Yes | 6 | 27.3% | 15 | 62.5% | 0.017a | 15 | 75.0% | 11 | 68.8% | 0.722 |
| No | 16 | 72.7% | 9 | 37.5% | 5 | 25.0% | 5 | 31.2% | |||
| Response Rc | Yes | 19 | 86.4% | 23 | 95.8% | 0.336 | 19 | 95.0% | 16 | 100.0% | 1 |
| No | 3 | 13.6% | 1 | 4.2% | 1 | 5.0% | 0 | 0.0% | |||
| Bleeding score | 0 | 0 | .0% | 3 | 12.5% | 0.224 | 0 | 0.0% | 3 | 18.8% | 0.020a |
| 1 | 19 | 86.4% | 16 | 66.7% | 15 | 75.0% | 13 | 81.2% | |||
| 2 | 3 | 13.6% | 5 | 20.8% | 5 | 25.0% | 0 | .0% | |||
| Cutaneous bleeding | Yes | 21 | 95.5% | 21 | 87.5% | 0.609 | 19 | 95.0% | 13 | 81.2% | 0.303 |
| No | 1 | 4.5% | 3 | 12.5% | 1 | 5.0% | 3 | 18.8% | |||
| Mucous membrane bleeding | Yes | 14 | 63.6% | 8 | 33.3% | 0.040a | 3 | 15.0% | 1 | 6.2% | 0.613 |
| No | 8 | 36.4% | 16 | 66.7% | 17 | 85.0% | 15 | 93.8% | |||
| Gynecological bleeding | Yes | 5 | 22.7% | 3 | 12.5% | 0.451 | 15 | 75.0% | 9 | 56.2% | 0.236 |
| No | 17 | 77.3% | 21 | 87.5% | 5 | 25.0% | 7 | 43.8% | |||
| Steroid therapy | Yes | 18 | 81.8% | 22 | 91.7% | 0.405 | 15 | 75.0% | 12 | 75.0% | 1 |
| No | 4 | 18.2% | 2 | 8.3% | 5 | 25.0% | 4 | 25.0% | |||
| Steroid dependence | Yes | 12 | 54.5% | 13 | 54.2% | 0.979 | 15 | 75.0% | 12 | 75.0% | 1 |
| No | 10 | 45.5% | 11 | 45.8% | 5 | 25.0% | 4 | 25.0% | |||
| Second-line treatment Azathioprine | Yes | 9 | 40.9% | 3 | 12.5% | 0.028a | 10 | 50.0% | 10 | 66.7% | 0.324 |
| No | 13 | 59.1% | 21 | 87.5% | 10 | 50.0% | 5 | 33.3% | |||
The analysis of the IL-4Rα A>G polymorphism within the cITP childhood onset group revealed a statistically significant association with disease severity and response to treatment, as carriers of the wild AA genotype are more susceptible to mucous membrane bleeding (p = 0.040), have a lower rate of CR (p = 0.017) and have a higher rate of second-line treatment (p = 0.028) than those with non-AA genotypes, however, they present at an older age than non-AA genotype carriers (p = 0.049) (Table 5).
DiscussionThe pathophysiology of ITP as an autoimmune disease is thought to include a complex interaction between genetic predisposition and environmental variables that results in immune response dysregulation. Only 60% of ITP patients have autoantibodies to the platelets GpIb/IX and GPIIb/IIIa, despite the fact that these autoantibodies have been well-documented to cause peripheral destruction and inhibit bone marrow production of platelets. This suggests that other immune mechanisms are involved in the pathogenesis.15
Auto-reactive T helper cells, which produce pro-inflammatory cytokines and help B cells to produce autoantibodies, are frequently involved in the pathogenesis of autoimmune disorders.16
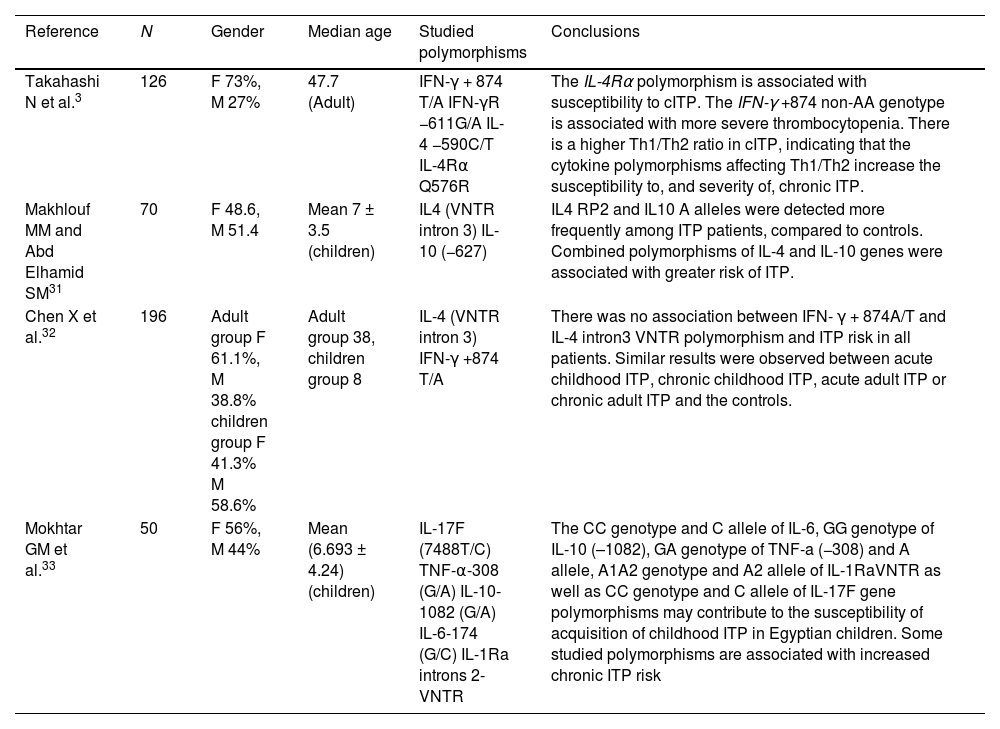
Numerous studies have linked inflammatory cytokine gene polymorphisms produced by Th1 cells to the development of ITP, but the role of gene polymorphisms related to Th2 cytokines was unknown until recently.17Table 6 summarizes some ITP studies involving gene polymorphisms of cytokines produced by T helper cells.
Important studies of the cytokine polymorphisms affecting Th1/Th2 in chronic ITP.
| Reference | N | Gender | Median age | Studied polymorphisms | Conclusions |
|---|---|---|---|---|---|
| Takahashi N et al.3 | 126 | F 73%, M 27% | 47.7 (Adult) | IFN-γ + 874 T/A IFN-γR −611G/A IL-4 −590C/T IL-4Rα Q576R | The IL-4Rα polymorphism is associated with susceptibility to cITP. The IFN-γ +874 non-AA genotype is associated with more severe thrombocytopenia. There is a higher Th1/Th2 ratio in cITP, indicating that the cytokine polymorphisms affecting Th1/Th2 increase the susceptibility to, and severity of, chronic ITP. |
| Makhlouf MM and Abd Elhamid SM31 | 70 | F 48.6, M 51.4 | Mean 7 ± 3.5 (children) | IL4 (VNTR intron 3) IL-10 (−627) | IL4 RP2 and IL10 A alleles were detected more frequently among ITP patients, compared to controls. Combined polymorphisms of IL-4 and IL-10 genes were associated with greater risk of ITP. |
| Chen X et al.32 | 196 | Adult group F 61.1%, M 38.8% children group F 41.3% M 58.6% | Adult group 38, children group 8 | IL-4 (VNTR intron 3) IFN-γ +874 T/A | There was no association between IFN- γ + 874A/T and IL-4 intron3 VNTR polymorphism and ITP risk in all patients. Similar results were observed between acute childhood ITP, chronic childhood ITP, acute adult ITP or chronic adult ITP and the controls. |
| Mokhtar GM et al.33 | 50 | F 56%, M 44% | Mean (6.693 ± 4.24) (children) | IL-17F (7488T/C) TNF-α-308 (G/A) IL-10-1082 (G/A) IL-6-174 (G/C) IL-1Ra introns 2- VNTR | The CC genotype and C allele of IL-6, GG genotype of IL-10 (–1082), GA genotype of TNF-a (−308) and A allele, A1A2 genotype and A2 allele of IL-1RaVNTR as well as CC genotype and C allele of IL-17F gene polymorphisms may contribute to the susceptibility of acquisition of childhood ITP in Egyptian children. Some studied polymorphisms are associated with increased chronic ITP risk |
The IL-4 enhances T helper cell differentiation toward Th2 and Th2 subsequently secretes additional IL-4, creating a positive feedback loop that further polarizes the immune response toward type 2 inflammation, which is primarily responsible for the pathogenesis of allergy and parasite disease. Nevertheless, a Th1/Th2 imbalance may be a significant factor in the pathogenesis of autoimmune diseases.18 Moreover, on some occasions the administration of IL-4 or generation of Th2 response during autoimmune inflammation may restore this balance.19 Guo et al. found that IL-4 levels decreased in patients with ITP and the Th1/Th2 balance was restored to a protective Th2 population after treatment with glucocorticoids.20
In the current study, we investigated the IL-4Rα A>G (rs1801275) SNP in a cohort of Egyptian cITP patients, as it was rarely investigated and documented, and we also studied clinical and laboratory variations between adulthood onset and childhood onset ITP.
In the current study, we found that the female gender was more affected in the adulthood onset group, which was statistically significant and matched several national and international studies,21,22 but was against some western population-based studies that confirmed the higher incidence of ITP in older men.23–25
Surprisingly, in our study, the adulthood onset group achieved higher maximum platelet response and CR more frequently than the childhood onset group, but they received second-line treatment more frequently, which was statistically significant, and this unexpected finding regarding CR closely matched a recent analysis of the 2-year follow-up data published by Schifferli et al., as they reported that, by the 24 months of follow-up among chronic ITP patients, 28% of the children and 30% of the adults had achieved remission.26
We assessed the distribution of genotype and allele frequencies of the IL-4Rα (A>G) SNP in cITP and the dominant and recessive models revealed no significant difference between Egyptian cITP patients and healthy controls. It was inconsistent with the first report of Takahashi et al., who documented that patients with cITP in a Japanese study had a significantly lower frequency of the IL-4Rα Q576R QQ genotype, compared to healthy controls, demonstrating that the IL-4Rα Q576R influences susceptibility to cITP,3 concluding that the Th17 polarization by the IL-4Rα Q576R polymorphism may affect the susceptibility to cITP.
Furthermore, when we stratified the cITP group according to the age of onset, no significant difference was detected between adulthood onset or childhood onset groups of cITP patients and healthy controls regarding genotype or allele frequencies. However, on stratification of cITP patients by gender, the analysis of the distribution of genotypes frequencies of the IL-4Rα (A>G) SNP, revealed that female patients had a statistically significant higher wild AA genotype and lower non-AA genotype than controls, which may influence susceptibility to cITP and explain the female predominance.
We explored the genetic association between the IL-4Rα A>G SNP and the severity of the cITP and we found that wild AA genotype carriers have more severe disease than non-AA genotype carriers, as AA genotype carriers had a higher bleeding score and 100% of the cases of severe bleeding were documented in carriers of the AA genotype. Furthermore, in the childhood onset group of cITP, carriers of the AA genotype were more susceptible to mucous membrane bleeding, had less incidence of achieving CR and had a higher incidence of receiving second-line treatment than carriers of non-AA genotypes and all these associations were statistically significant.
The functional assay performed by Hershey et al. has shown that the substitution of glutamine by arginine at the cytoplasmic tail of the IL4Rα subunit (position 576) was associated with enhanced signaling of the IL-4 receptor26 and, thus, the non-AA genotypes may be associated with increased activity of the IL4, enhancing the balance of Th1/Th2 towards Th2, while the AA genotype may be associated with decreased activity of the IL4 and the down-regulation of type I inflammation. This could explain the association of the more sever cITP in the AA genotype, as shown in our study, and is also supported by the finding of decreased serum levels of the IL-4 in the cITP, as reported in a few studies.27–28
The IL-4Rα (rs1801275) A>G polymorphism was rarely studied in ITP, but has been studied in other autoimmune diseases, for example, rheumatoid arthritis (RA), and revealed that the mutant allele is protective against autoimmunity in females. One such study was conducted on Egyptian females and concluded that females carrying a mutant allele are protected from developing erosive RA, while carriers of the wild allele were significantly more likely to develop severe RA.29 Another study was conducted on Saudi females and revealed that the wild genotype carriers develop significantly higher levels of rheumatoid factor and a severe form of RA.30 This study could also support that decreased IL-4 activity in the wild allele is associated with more severe disease.
The discrepancy between the results of our study and that of Takahashi et al. could be attributed to differences in ethnic origins of the studied populations or the effect of the contribution of other polymorphisms that may present in the IL-4R gene, but to our knowledge, it is the first report demonstrating that the IL-4Rα (rs1801275) A>G polymorphism influences the severity of the cITP. The sample size was one of the main limitations of our study, so further studies on a larger group of cITP patients are required to confirm the current conclusion.
ConclusionThe IL-4Rα (rs1801275) A>G polymorphism may affect the clinical severity of the cITP and treatment response in the Egyptian population, as homozygous wild AA genotype carriers are significantly susceptible to more severe disease than non-AA genotype carriers.
DeclarationsEthics approval and consent to participate: approved by the local Ethical Committee of the Internal Medicine Department, Faculty of Medicine, Cairo University.
Consent for publication: Not applicable.
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
Authors' contributionsThe idea for the research was presented by Prof. Dr. NT and Dr. MAM and data collection was performed by all authors, Meticulous laboratory work was performed under the supervision of Dr. ND. The paper was written by Dr. ND & Dr. DM and all the work was revised by all authors with an equal contribution.