Beta-thalassemia major patients need a regular blood transfusion to have an initial normal growth. However, these patients have an increased risk of developing alloantibodies. Our main goal was to study HLA alloimmunization in Moroccan Beta-thalassemia patients by confronting it with transfusion and demographic criteria, exploring the involvement of HLA typing profile in the development of HLA antibodies and in turn determining risk factors for their development.
MethodsThe study consisted of 53 Moroccan pediatric patients with Beta-thalassemia major. Screening for HLA alloantibodies was performed using Luminex technology Whereas HLA genotyping was done with sequence-specific primers (PCR-SSP). Results: In this study, 50.9% of patients have been identified as positive for HLA antibodies, with 59.3% having both HLA Class I and Class II antibodies. A significant increase frequency of DRB1*11 allele was revealed in non-immunized patients (34.6% vs. 0%, p = 0.001). Our results also revealed that the majority of our HLA immunized patients were women (72.4% vs. 27.6%, p = 0.001), and transfused with more than 300 units of RBC units (66.7% vs. 33.3%, p = 0.02). There were statistically significant differences when comparing these frequencies.
ConclusionsThis paper revealed that the transfusion dependent Beta-thalassemia major patients are exposed to risk of developing HLA antibodies following transfusions with leukoreduced RBC units. The HLA DRB1*11 was a protective factor against HLA alloimmunization in our beta-thalassemia major patients.
Beta-thalassemia major (β-TM) is a genetic blood disorder characterized by a deficiency in the beta-globin chain synthesis and manifests as severe anemia from early childhood.1 The β-TM patients need a regular blood transfusion. The transfusion therapy usually starts at an early age, allowing β-TM patients to have a normal initial growth, and continues for life.2 However, these patients have an increased risk of developing antibodies (Abs) against red blood cells (RBCs), human platelet antigens, and human leukocyte antigens (HLAs).3–8 The focus on alloimmunization rates has increased, particularly with the advent of allogeneic hematopoietic stem cell transplantation (HSCT) as a definitive therapy for patients with β-TM.8,9 In literature, several studies have shown that these antibodies have been related to immune-mediated refractoriness to platelet transfusion during post-hematopoietic stem cell transplantation (HSCT) aplasia and increases the risk of rejection of hematopoietic stem cells (HSCs).4,10–13 Therefore, the detection of susceptible responders before the blood transfusion process can play a useful role in preventing alloimmunization.
In practice all β-TM patients benefit from a screening for RBC Abs before and after each transfusion as part of a systematic monitoring plan for multi-transfused patients. No such test is systematically performed for Abs HLA.14,15 Little information is available regarding the prevalence and risk factors of HLA alloimmunization in β-TM patients. To our knowledge, the data in this context remain unknown in the Moroccan population. Here, we have studied the frequency of HLA Abs in Moroccan β-TM patients and determined the transfusion and demographic characteristics which expose patients to HLA alloimmunization. Using molecular typing, we have also compared the distribution and the frequencies of the HLA-DRB1* and DQB1* alleles in immunization and non-immunization β-TM patients.
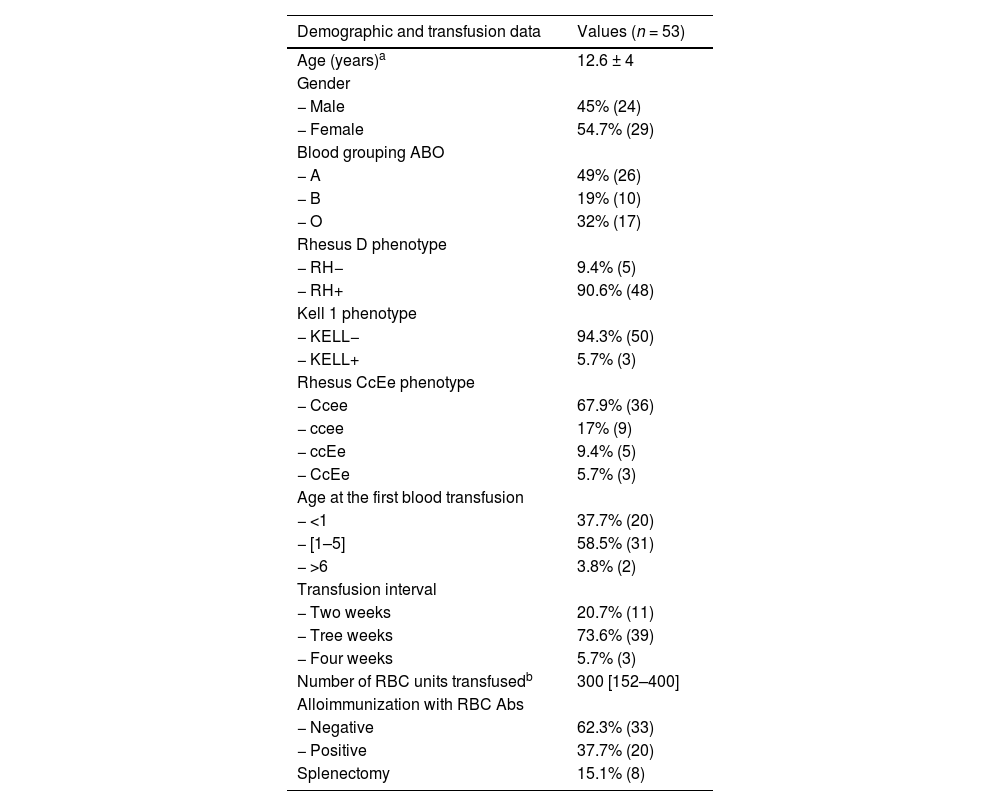
MethodThe study included 53 unrelated Moroccan patients with β-TM receiving treatment and follow-up at the pediatric oncology center of the Children's Hospital in Rabat. Table 1 summarizes the demographic and transfusion characteristics of patients collected from medical records. They received a regular blood transfusion using RBC units that were always leukoreduced and phenotypically adapted for the Rh and KELL systems. This procedure was applied since the start of transfusion regimen. All subjects gave their informed written consent for anonymous collection of data for research purposes. All analyses were performed in the laboratory of immune-histocompatibility of the Ibn Sina University Hospital of Rabat.
Demographic and transfusion data of Moroccan β-TM patients.
| Demographic and transfusion data | Values (n = 53) |
|---|---|
| Age (years)a | 12.6 ± 4 |
| Gender | |
| − Male | 45% (24) |
| − Female | 54.7% (29) |
| Blood grouping ABO | |
| − A | 49% (26) |
| − B | 19% (10) |
| − O | 32% (17) |
| Rhesus D phenotype | |
| − RH− | 9.4% (5) |
| − RH+ | 90.6% (48) |
| Kell 1 phenotype | |
| − KELL− | 94.3% (50) |
| − KELL+ | 5.7% (3) |
| Rhesus CcEe phenotype | |
| − Ccee | 67.9% (36) |
| − ccee | 17% (9) |
| − ccEe | 9.4% (5) |
| − CcEe | 5.7% (3) |
| Age at the first blood transfusion | |
| − <1 | 37.7% (20) |
| − [1–5] | 58.5% (31) |
| − >6 | 3.8% (2) |
| Transfusion interval | |
| − Two weeks | 20.7% (11) |
| − Tree weeks | 73.6% (39) |
| − Four weeks | 5.7% (3) |
| Number of RBC units transfusedb | 300 [152–400] |
| Alloimmunization with RBC Abs | |
| − Negative | 62.3% (33) |
| − Positive | 37.7% (20) |
| Splenectomy | 15.1% (8) |
RBC: red blood cell; Abs: antibodies.
The sera from β-TM patients were tested with the LABScreen antigen bead assay using the LABScreen mixed kit (One Lambda Canoga Park, CA, USA) according to the manufacturer's protocol. The plate fluorescence was read using the Luminex 3D. The results were analyzed using Fusion 3.0 software (One Lambda). Acceptance criteria for the sample result was negative control (NC) bead MFI < 300, positive control (PC) bead MFI > 1500 and PC/NC ratio > 2.0. The positivity threshold was set at a ratio > 3.
DNA extraction and molecular genotypingThe β-MT Patients were typed by a molecular method for HLA class II (-DRB1* and -DQB1*). The genomic deoxyribonucleic acid (DNA) was first extracted and purified from peripheral blood samples collected in 5% ethylenediamine tetraacetic acid (EDTA) using a commercial kit (Qiagen). The HLA-DRB1* and -DQB1* typing was performed by polymerase chain reaction-sequence specific primers (PCR-SSPs) using micro generic HLA DNA typing trays (One Lambda) according to the manufacturer's protocol. Analysis of the allele typing was archived by the HLA Fusion v. 3.0 software (One Lambda).
Statistical analysisThe HLA Abs frequency and p-value were determined using the commercial SPSS software (V13). The HLA allele, demographic and transfusional data frequencies were compared between alloimmunized and non-alloimmunized β-TM patients using the Pearson's chi-squared test or the Fisher's exact test, as appropriate. Furthermore, univariate and multivariate logistic regression analyses were performed to identify the factors associated with HLA immunization. All independent variables with P-value < 0.20 in the univariate analysis were taken into account in the multivariate logistic regression analysis. A p-value less than 0.05 was considered statistically significant.
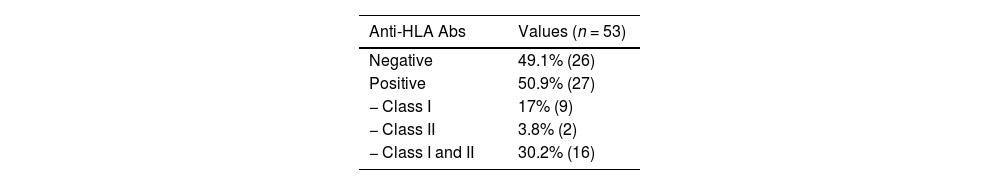
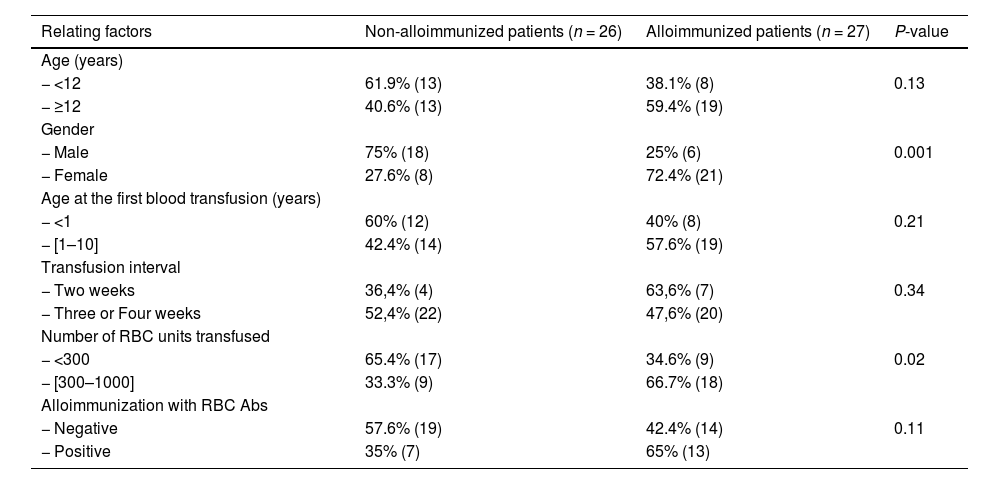
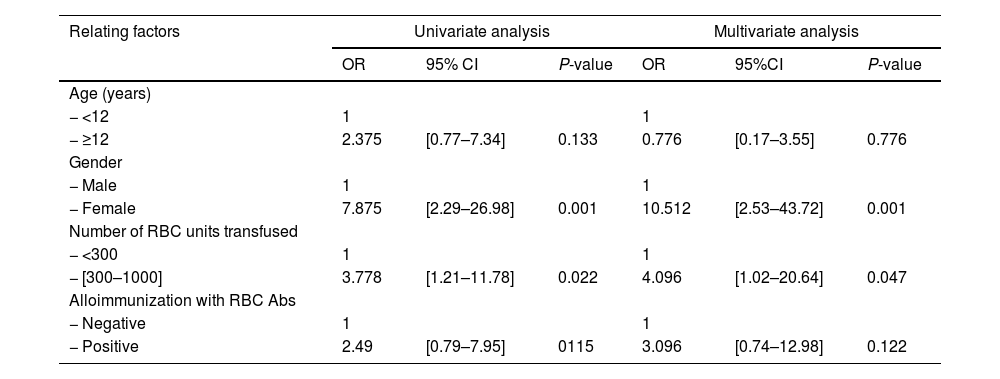
ResultFrequency of HLA ABS in β-TM patientsFifty-three Moroccan β-TM patients have been screened for HLA Abs. 50.9% (27) were tested positive. Of these alloimmunization patients, 59.3% (16/27) had class I and class II anti-HLA Abs (Table 2). Comparing the two groups showed that immune HLA patients were adolescents in 59.4% versus 40.6% for non-immune patients. However, this difference was not statistically significant (p = 0.13). The finding also found that immune HLA patients were in the majority of women (72.4% versus 27.6%, p = 0.001) and transfused with more than 300 units of RBC in 66.7% as against 33.3%, p = 0.02. There were statistically significant differences when comparing these frequencies. 65% of HLA immunized patients were positive for anti-RBC antibodies against 35% for non-HLA immunized patients. this result was not statistically significant (p = 0.11). (Table 3). Multivariate analysis has showed that the factors associated with HLA immunization were female (OR = 10.512; 95%CI: [2.53-43.72]) and the number of transfused CGR >300 (OR = 4. 4.096; 95%CI: [1.02-20.64]) (Table 4).
Relation between different variables and their correlation to results of anti-HLA Abs screening in β- TM patients.
Abs : antibodies ; n : number of individuals; RBC : red blood Cell, P-value : significance level.
Univariate and multivariate analysis of HLA alloimmunization in the β- TM patients.
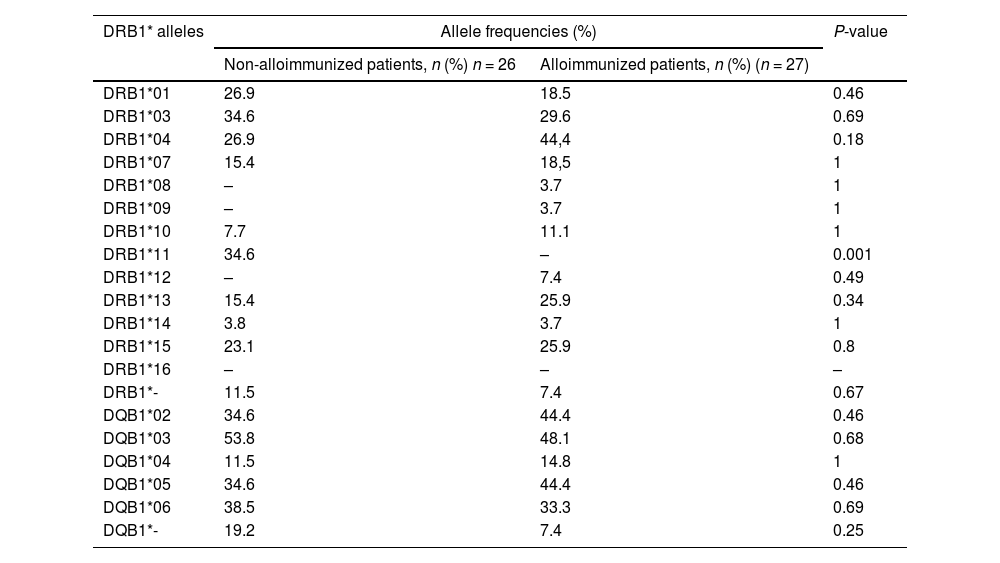
In the immunized patients, we found eleven alleles, with the exception of DRB1*11 and DRB*16 which were absent. HLA- DRB1*04(44.4%) DRB1*13 (25.9%) and DRB1*15 (25.9%) alleles were more common in this group. Nine HLA-DRB1* alleles have been identified in non-immunized patients. The most common were HLA-DRB1*03 (34.6%) and DRB1*11 (34.6%). The comparison of the two groups revealed a significant increase frequency of DRB1*11 allele in non-immunized patients (34.6% vs. 0%, p = 0.001). We have identified five alleles at the HLA-DQB1 locus in both groups. The high frequency was observed for DQB1*02(44.4%) and DQB1*05 (44.4%) alleles in immunized patients. However, the difference was no statistically significant between non-immunized and immunized patients (Table 5).
HLA-DRB1* and DQB1* allele frequencies in alloimmunized and non- alloimmunized β-TM patients with anti-HLA Abs.
HLA: human leucocyte antigen; n: number of individuals; P-value: significance level.
Alloimmunization is one of the major complications of a regular blood transfusion in β-TM patients.3,5,16 Several studies have investigated the prevalence of RBC Abs, but few have focused on HLA Abs. In our study, the frequency of HLA Abs was 50.9% in β-TM patients. There was higher than that reported by previous studies on HLA alloimmunization in β-TM (30% to 42%).5,6,17 The authors were limited in HLA Abs screening to the lymphocytotoxicity test (LCT) or enzyme-linked immunosorbent assay (ELISA), which are less sensitive than the Luminex. In contrast, the result was similar to that of the study conducted by Lo et al. (54%) and of another by McPherson Yee et al. (53%). They had used ELISA and Luminex, respectively, to demonstrate HLA Abs.5,18 In Middle East β-TM patients, 82% tested positive for HLA Abs by Luminex. This high level of HLA Abs was related to the transfusion with non-leukodepleted RBCs.4 Numerous clinical trials have shown that the use of leukoreduced blood units reduced HLA alloimmunization with an average of 15% to 40%.18 The HLA alloimmunization can be explained by residual white blood cells and platelets in blood components. Our patients had systematically received leukoreduced RBCs. We used the technology of leukocyte reduction by filtration, before or after storage. The amounts of white blood cells (WBCs) and platelets in leukodepleted RBCs were approximately 7 × 106 per unit. Exposure to such important levels of residual WBCs may explain the high frequency of HLA immunization in our chronic transfused patients.
HLA alloimmunization is one of the major detrimental effects of transfusion, and concerns patients with frequent transfusion needs. In sickle cell disease (SCD), HLA alloimmunization has been reported to occur in 34% of the patients. This low rate was related to the use of transfusion only when SCD crises happen. On the other hand, SCD patients experience a decline or downright a loss of HLA Abs as the time since the immunological conflict during the crisis increases.19
Patients who have developed HLA Abs are prone to febrile non-hemolytic transfusion reactions. In addition, they can also become difficult matches for solid organ transplantation.20–22 In these settings, donor-specific HLA Abs may further increase the risk of rejection, while class I HLA Abs may cause platelet transfusion refractoriness prior to engraftment.23
Our results showed that 92.6% HLA immunized b-TM patients were positive for class I (33.3% had class I anti-HLA Abs only and 59.3% had both class I and class II anti-HLA Abs). The same result was reported in the population who received leukoreduced blood without irradiation.18 Philip et al. reported the rate of 30% HLA class I immunization in patients who had received leukoreduced, irradiated fresh blood.6 Irradiation of the blood destroyed residual WBCs, which normally reduce immunization against HLA class I and prevent immunization against HLA class II. Patients may demonstrate a loss of HLA antibody as the time from the immunologic challenge lengthens. Various risk factors for alloimmunization in β-TM patients have been reported in the literature.3,24 It appeared necessary to identify patients likely to develop alloantibodies. Some authors have found a decreased risk of alloimmunization in patients who received their first transfusion before three years. Transfusions before this age have been shown to offer some protection against alloimmunization in patients with b-TM.25–27 This fact would be due to the immune tolerance induced by early exposure to repeated transfusions. Other risk factors that have been reported are the frequency of transfusions and the cumulative number of units transfused, which reflect higher exposure and longer duration of transfusion.28–30 This study found no influence of age, gender, age at the first blood transfusion, transfusion interval, number of RBC units transfused or alloimmunization with RBC Abs of the patient, in agreement with Ben Salah et al.6,16 In our study, subjects with HLA Abs tended to be older, This study showed that HLA immunization was associated with gender and number of RBC units transfused. However, we didn’t find any influence of age, transfusion interval, age at the first blood transfusion or RBC immunization. This goes in agreement with Ben Salah et al.’s study results.6,16
In the case of SCD, the direct association between RBC antigens and HLA antibody responses is demonstrated in several studies carried out in different populations.31 In SCD, the prevalence of RBC alloimmunization is the highest among polytransfused patients, with a rate up to 75%.3233 This can be explained mostly by the fact that SCD patients often have ongoing inflammation associated with SCD crisis.19 It is important to note that the majority of RBC units received by these patients were leukodepleted. In our study, subjects with HLA Abs tended to be older, were predominantly females and were alloimmunized with RBC Abs. Despite the many foreign antigens that patients with β-TM are exposed to, some individuals do not develop antibodies. Besides an environmental condition, genetic factors can also play a role. Genetic studies have examined the role of HLA-DRB1* alleles on the development of alloantibodies. They have shown that HLA-DRB1* alleles can enhance antibody formation in individuals with particular phenotypes.34 In fact, the differences in the groove peptide among the HLA-DRB1* alleles cause the presentation of foreign antigens to the immune cell and the induction of alloimmunization in some patients. Verduin et al. have been tested the association with the development of HLA Abs in β-TM patients and the DRB1*15 allele. They have reported that the DRB1*15 allele had a direct association with the HLA alloimmunization.35 In our study, the frequency of HLA-DRB1*15 was higher in immunized β-TM patients but not significantly (25.9% vs. 23.1%, p = 0.8). Not having obtained a significant result can be explained by the difference in the distribution of HLA genes between populations, but in our study, it is probably related to the size of the sample. No other predisposing allele was identified, however, HLA DRB1*11 was a protective factor against HLA alloimmunization in our β-TM patients (34.6% vs. 0%, p = 0.001).
ConclusionHalf of our patients had HLA Abs following transfusions with leukoreduced RBC units. For these patients, identification of HLA Abs will be useful if subsequent organ or HSCT is indicated. Knowledge of the factors related to the HLA alloimmunization can significantly assist in the selection process before transfusion and, therefore, decrease this complication. No risk factors have been identified, but the DRB1*11 may play an important role in protecting against the HLA alloimmunization. Further studies with larger samples, with the identification of HLA Abs and the study of the kinetics of HLA Abs, will be essential to develop evidence-based guidelines.
Sources of supportThere were no sources of support for this study in the form of grants, equipment, or drugs.
CRediT authorship contribution statementSanae Ouadghiri: Conceptualization, Formal analysis, Writing – original draft. Kaoutar El Morabit: Investigation, Writing – original draft. Naoual Elansari: Investigation, Writing – review & editing. Ouafae Atouf: Conceptualization, Writing – review & editing. Maria Elkababri: Writing – review & editing. Laila Hessissen: Writing – review & editing. Malika Essakalli: Writing – review & editing.