Diversity in Classical Hematology Research
More infoThe Band 3 is a red blood cell protein that carries the Dia and Dib antigens from the Diego blood system. The SLC4A1 gene encodes Band 3; Band 3 Memphis is a polymorphism of normal Band 3 and has two variants, but only the variant II carries the Dia antigen.
ObjectivesDescribe the frequencies of the DI*A and DI*B alleles and the Band 3 Memphis among blood donors, sickle cell disease (SCD) patients and Amazonian Indians.
MethodsA total of 427 blood samples were collected and separated into three groups: 206 unrelated blood donors, 90 patients with SCD and 131 Amazonian Indians. We performed DI*A/B, normal Band 3 and Band 3 Memphis genotyping, using the Polymerase Chain Reaction Restriction Fragment Length Polymorphism (PCR–RFLP).
ResultsThe frequency of the DI*A/DI*A genotype was 0.5% in blood donors and it was not found in other groups. The frequency of the DI*A/DI*B was higher in Amazonian Indians (33.6%) and the frequency of the DI*B/DI*B was highest in blood donors (92.2%). All 105 individuals tested were positive for the presence of normal Band 3 and of these individuals, only 5/105 (4.8%) presented the Band 3 Memphis mutation.
ConclusionWe observed a higher frequency of the DI*B allele in blood donors and a low frequency of the DI*A/DI*A genotype in all groups studied. The Band 3 Memphis was found in a higher frequency in the blood donor group. Our findings highlight the importance of analyzing different population groups to gain a better understanding of the genetic association of blood group antigens.
The Diego blood group system is composed of 22 antigens located in the Band 3 protein present in the membrane of red blood cells (RBC). It is an integral protein in the membrane of RBCs, carries anions HCO3−, Cl− and SO4−through the membrane and has a molecular mass of 95 kd and two distinct domains: the N-terminal cytoplasmic domain (40-kd) and the C-terminal cytoplasmic domain (55-kd). The gene encoding Band 3 is SLC4A1, AE1 ("solute carrier family 4, anion exchanger, member 1 gene"). This gene has 18 kb, in a sequence organized in 20 exons and 19 introns, and it is located in chromosome 17, region q12-q21.1,2
The Band 3 Memphis is a polymorphism of the Band 3 protein that results in a point mutation (166A>G) in the SLC4A1, leading to an amino acid substitution Lys56Glu. Furthermore, the Band 3 Memphis has two variants (I and II), which are distinguished by their susceptibility to covalent bonding with 4,4′-diisothiocyanato-1,2-diphenylmethane-2,2′-disulphonic acid (H2DIDS). The Dia antigen is reported to be associated with the Band 3 Memphis variant IIx.3,4,5
Other SLC4A1 studies stated that the Diego polymorphism results from a point mutation (2561T>C) at Exon 19, leading to a single amino acid substitution at position 854 (Leu-for Dia and Pro-for Dib). In addition, it was confirmed that the Band 3 Memphis II is linked to the Dia antigen. However, some analyses revealed that the Dia antigen does not have a strict association with the 166A>G.5,6
The Dia antigen (DI*01 allele) is considered a Mongoloid factor and the frequency is variant, according to the region studied, and previous serologic and molecular studies have shown that the Dia antigen is found in Amazonian Indians (5 - 65%), Japanese (12%), Chinese (5%), people from the southwest region of the state of Paraná, Brazil (0.4%), while it is rare among Caucasians and Africans. The Dib antigen has a high prevalence in all populations studied.5,7,8
Dia antigen incompatibility may cause hemolytic transfusion reactions and hemolytic disease in the newborn.9,10 This article describes the allele frequencies of the DI*A and DI*B and the associated incidence of the Band 3 Memphis among Brazilians.
MethodsStudy populationA total of 427 blood samples were collected from Brazilian individuals after obtaining a signed informed consent (following the Research Ethics Committee requirements). The samples were separated into three groups: (1) 206 unrelated blood donors, (2) 90 Afro-Brazilian patients with sickle cell disease (SCD) and (3) 131 Amazonian Indians (from the Xikrin tribe).
The Xikrin Indians belong to the northern Kayapo region and are descendants of the Jê language family. It is a small tribe established on the left riverside of the Cateté River, in the State of Pará (Northern Brazil). The Xikrin of Cateté is a highly homogeneous population with related individuals.
This study was approved by the Research Ethics Committee of the Universidade Federal de São Paulo (CEP 1226/08).
Genomic DNA extractionThe DNA extraction was performed from 10 mL of whole blood from samples collected with the anticoagulant ethylenediaminetetraacetic acid (EDTA), using the phenol-chloroform method11 and DNAzol (Gibco BRL®, Gaithersburg, MD), according to the manufacturer's recommendations.
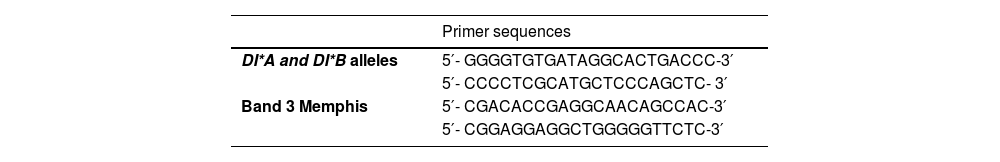
DI*A, Di*B and Band 3 Memphis genotypingThe polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method was performed as described by Orita et al.12 The PCR assay was designed to amplify the sequences of the DI*A, DI*B alleles and Band 3 Memphis with specific primers, as shown in Table 1. The PCR amplification was performed, using 5.0µL of the buffer, 200 ng genomic of DNA, 1.5 mM of MgCl2, 0.2 mM of dNTPs, 2.5 U Taq DNA polymerase, and 0.5 μM of specific primers. After PCR amplification, the products were analyzed by electrophoresis, using 2.0% agarose gel.
Analyses with the appropriated enzymes were used to identify the DI*A and DI*B polymorphisms in exon 19 and Band 3 Memphis in exon 4.6,14 The restriction enzyme Mspl (20.0 U) was used to distinguish the DI*A and DI*B polymorphisms, using the PCR product, and the restriction enzyme Mnll (5.0 U) was used to obtain the Band 3 Memphis, using the PCR product. Both reactions were incubated at 37 °C for 16 h and frozen to −20 °C for subsequent Low Melting Point electrophoresis in 2.0% agarose gel.
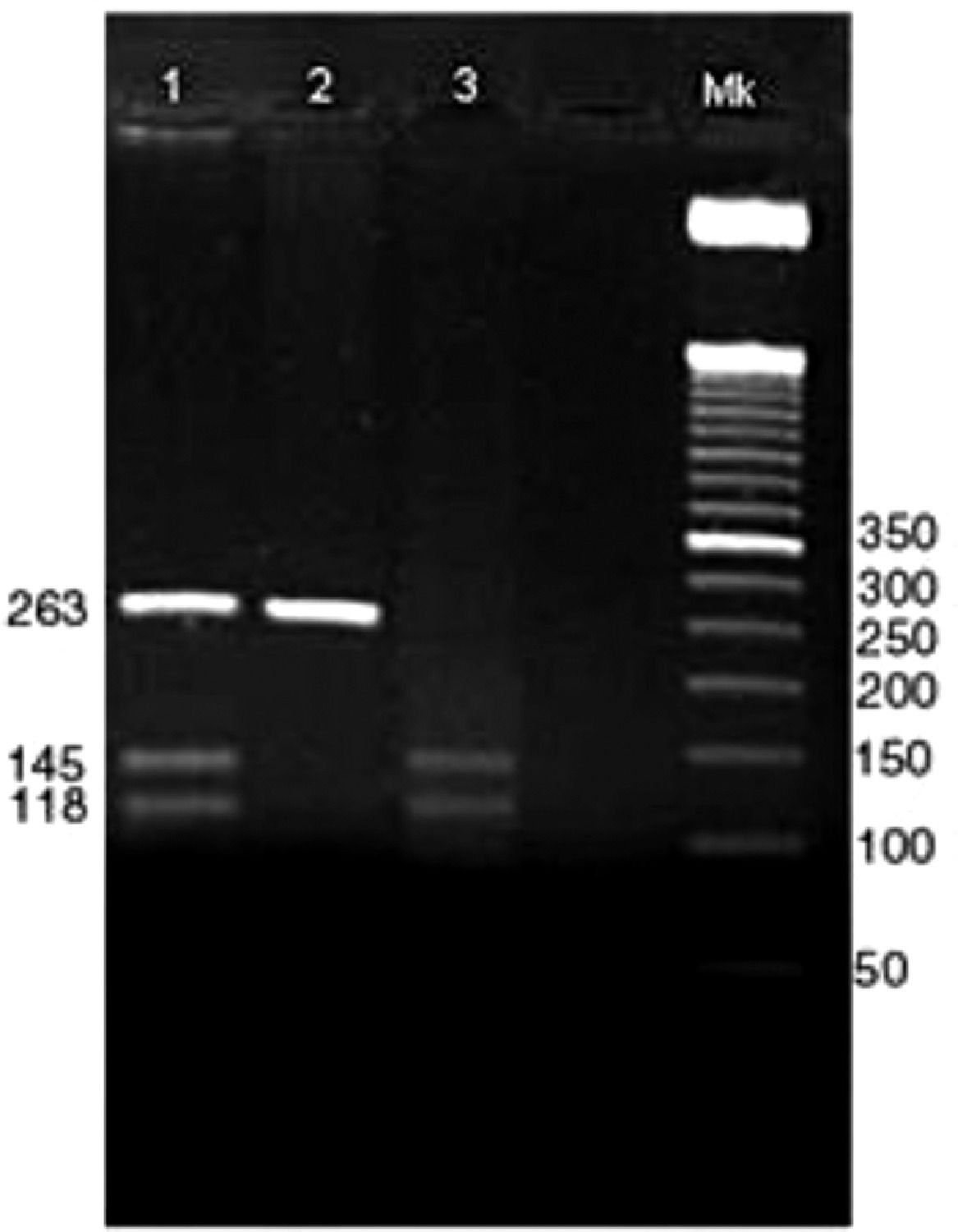
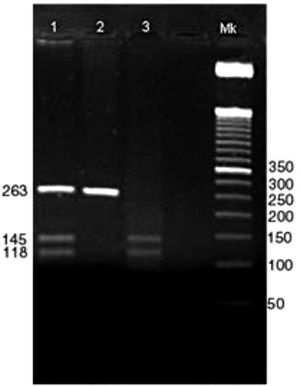
Positive bands with 263 bp showed the presence of the DI*A allele, positive bands with 145 and 118 bp showed the presence of the DI*B allele. Positive heterozygous bands are digested in 263, 145 and 118 bp (Figure 1).
Genotyping of DI*A and DI*B alleles.
Columns:
1 → Amplification of PCR and digestion, respectively, of the heterozygous individual for the DI*A and DI*B alleles.
2 → Amplification of PCR and digestion, respectively, of the homozygous individuals for the DI*A allele.
3 → Amplification of PCR and digestion, respectively, of the homozygous individual for the DI*B allele.
Mk → Marker of 50 bp.
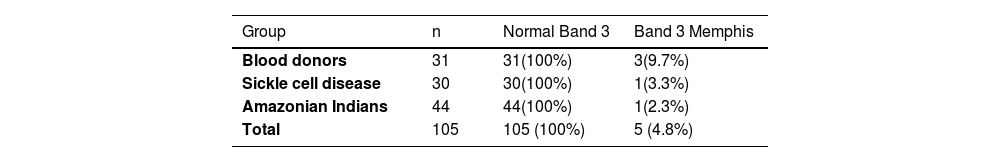
Normal Band 3 and Band 3 Memphis genotyping was performed in 105 individuals included in the present study: 31 Brazilian blood donors, 30 SCD patients and 44 Amazonian Indians.
Statistical analysisStatistical analysis was performed using the "R program" (Vienna, Austria), version 3.4.2. The Qui-Square test and the exact Fisher test were performed to compare proportions. A p-value of less than 0.05 was considered significant.
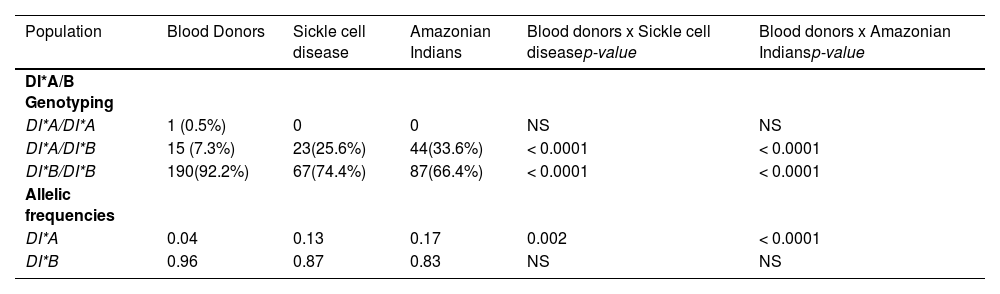
ResultsAs shown in Table 2, the genotyping results showed that, of 206 blood donors, only one (0.5%) individual was homozygous for the DI*A allele, 15 (7.3%) individuals were heterozygous for the DI*A and DI*B alleles and 190 (92.2%) individuals were homozygous for the DI*B allele. Of the 90 patients with SCD, none were homozygous for the DI*A allele, 23 (25.6%) were heterozygous for the DI*A and DI*B alleles and 67 (74.4%) were homozygous for the DI*B allele. Of the 131 Amazonian Indians, 44 (33.6%) were heterozygous for the DI*A/*B alleles and 87 (66.4%) were homozygous for the DI*B allele (Table 2).
Diego genotyping and Allelic frequencies.
NS, no significance.
A statistically significant result was found upon comparing blood donors with the other two groups (SCD and Amazonian Indians). The DI*A/DI*B genotype was more frequent in SCD patients and Amazon Indians in comparison to blood donors (p < 0.0001). An inversely proportional result was found upon comparing the frequency of the DI*B/DI*B genotype in the groups studied, the main frequency of blood donors of this genotype, compared to SCD patients and Amazonian Indians (p < 0.0001).
The DI*A allele was more frequent in SCD patients and Amazonian Indians, compared to blood donors (p = 0.002 and p = < 0.0001, respectively).
We analyzed 105 individuals for the presence of normal Band 3 and Band 3 Memphis. All individuals included in this analysis showed positive results for the presence of normal Band 3 (n = 105) and only five (4.8%) subjects presented the Band 3 Memphis mutation: 3/31 (9.7%) blood donors, 1/30 (3.3%) SCD patients and 1/44 (2.3%) Amazonian Indians (Table 3).
DiscussionThe present study identified a higher frequency of the DI*B allele in blood donors and a low frequency of the DI*A/DI*A genotype in all studied groups. The Band 3 Memphis was found in a higher frequency in the blood donor group.
We observed that the presence of the DI*A/DI*B heterozygosis in Amazonian Indians (33.6%) is similar to the results observed in Native American individuals (36%) and the lower frequency of homozygous individuals for the DI*A allele confirms the results described in other studies.5,7,8 Furthermore, we observed a greater proportion of Amazonian Indians with the DI*A/DI*B genotyping (33.6%), compared to blood donors (7.3%), corroborating the results obtained in previous studies.5,13 The distribution of the DI*A and DI*B allele and genotype frequencies in Brazilian blood donors and SCD patients indicates a possible influence of the mixed Brazilian population on its descendants and on the gene flow.
Knowledge of the frequency of blood group antigens and alleles in distinct populations and ethnic groups is useful in transfusion and obstetrics medicine because RBC antigen incompatibilities are responsible for alloantibody formation.14 Previous studies showed that the prevalence of the Dia antigen presents racial variation; in a study with blood donors in Ecuador, a 25% prevalence of the Dia antigen and an alloimmunization frequency of 6.09% against this antigen were found and a similar alloimmunization frequency was found in a Brazilian population study, which reported an anti-Dia alloantibody prevalence of 6.0% in 12,904 multi-transfused patients.15,16 The presence of the Dia antigen in blood donors poses a risk to the Dia negative-transfused patients.
Anti-Dia alloantibodies may cause hemolytic transfusion reactions, hemolytic disease of the fetus and newborn and have been detected after 30 years of historical alloimmunization.10,13,17 Thus, in Brazil, the use of Dia positive cells in antibody screening is of paramount importance.
ConclusionWe analyzed the presence or absence of the Band 3 Memphis and found signs of polymorphism in three blood donors, one Amazonian Indian and one SCD patient. The results revealed a greater presence of the Band 3 Memphis in blood donors, compared to the other groups analyzed; however, it would be interesting to perform a new study with a greater number of individuals to determine whether it was just a finding or if it is related to some regional characteristic. Mutations in other genes can cause changes in Band 3; an example is an SCD alteration in which the increased oxidation causes accelerated aging of Band 3 and increased IgG binding and cellular removal.18,19 Furthermore, the study showed that in the non-human primates tested, Band 3-Memphis (56Glu) and Dib (854Pro) are present, suggesting that at these polymorphic sites, this is the ancestral SCLA4A1.20
AcknowledgementsAuthorship- •
Authors who performed the research: Alessandra Kaliniczenko, Akemi Kuroda, Chiba, José O. Bordin
- •
Authors who designed the research study: Alessandra Kaliniczenko, Akemi Kuroda Chiba, João P. B. Vieira Filho, José O. Bordin
- •
Authors who contributed essential reagents or tools: Alessandra Kaliniczenko, Akemi Kuroda Chiba, João P. B. Vieira Filho, José O. Bordin
- •
Authors who analyzed the data: Alessandra Kaliniczenko, Bruno Ribeiro Cruz, Juliana Oliveira Martins, José O. Bordin
- •
Authors who wrote the paper: Alessandra Kaliniczenko, Bruno Ribeiro Cruz, Juliana Oliveira Martins, Flavia Gehrke
- •
Authors who revised the paper: Alessandra Kaliniczenko, Bruno Ribeiro Cruz, Juliana Oliveira Martins
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), which granted the scholarship and financial aid that fostered this study.