Hematopoietic stem cell transplantation (HSCT) is a treatment that requires long periods of hospitalization. The mobility restrictions result in physical, functional and psychological impairments. Physical exercise is a therapy that can restore physical and functional capacities; however, it is necessary to understand the effects of its practice in post-HSCT individuals. The purpose of this systematic review (SR) was to assess the impact of physical exercise in children and adolescents undergoing HSCT. The SR was conducted following the PRISMA guidelines through search in the electronic databases Embase, Lilacs, PEDro, PubMed and SCOPUS, without limitation of dates and languages. Randomized or non-randomized clinical trials with children and adolescents who underwent HSCT, aged between 3 to 19 years old, who participated in a regular physical activity program, were assessed. After removing duplicates and selecting studies according to the eligibility criteria, seven parallel studies incorporating hospitalized and discharged participants undertaking aerobic and strengthening exercises were included in this study. The main outcomes analyzed were exercise capacity, quality-of-life, body composition and freedom. Five studies comprised the meta-analysis regarding the effects of the distance walked in the 6-min walk test and quality-of-life. Physical exercise is considered to be safe, feasible and efficacious to prevent the decline of the quality-of-life in children and adolescents undergoing HCST, as well as a considerable improvement in physical capacity.
Hematopoietic Stem Cell Transplantation (HSCT) has become the most appropriate method to treat leukemia at diagnosis and for patients that relapse. It is considered a significant therapeutic modality for a large number of children with malignant and non-malignant diseases and is performed to replace bone marrow that is damaged, restoring its production of new blood cells and promoting the growth of new marrow.1–5
The HSCT is a potentially life-saving procedure, but it is still associated with significant transplant-related mortality rates. The complexity involving chemotherapy toxicity and physical inactivity experienced by those undergoing HSCT place individuals at risk of developing long-term complications, such as musculoskeletal disorders and cardiorespiratory (CRP) compromise. Lack of pre-transplant conditioning protocols may intensify the effects of motor and respiratory repercussion.2,5,7
A continuous loss of skeletal mass (with or without loss of fat mass) leads to progressive motor impairment. It results in lack of physical activity, loss of muscle strength, which may be worsened when there is myopathy or atrophy of skeletal muscles due to the use of systemic corticoids. This functional decline and deconditioning ultimately impacts the ability to participate in daily living activities and additionally, the resulting increments in the metabolic rate and energy consumption produce tiredness and fatigue. Alterations in the respiratory capacity may also be observed due to the characteristic abnormality of respiratory mechanisms, leading to changes in minute volume, tidal volume and strength.5,7
Thereat, an exercise program (EP), aims to prevent complications of bedrest in order to restore and maintain physical and respiratory function, consequently improving muscle strength. Generally, it includes graduated mobilization to perform out-of-bed activities, joint range-of-motion exercise, breathing exercises to prevent pulmonary complications, muscle stretching, strengthening training, core stability exercises and endurance training, as indicated.5–7
It is known that exercise rehabilitation programs play an essential part in enabling patients to regain the confidence to resume post-transplant activities. For example, exercise training program are generally considered the cornerstone of the multidisciplinary program in some diseases, such as cardiac heart failure or chronic obstructive pulmonary disease. As cancer is transitioning into a “chronic disease”, there is now a push to implement strategies that address the associated morbidity. All considered, a better understanding of exercise relevance and effects among bone marrow transplantation patients may improve exercise training deliverance for these individuals, consequently improving physical post-transplant performance, preventing pulmonary complications and promoting the utmost health-related quality-of-life (QoL).
Thus, the present study aimed to describe the effects of physical exercise on children and adolescents who had undergone HSCT.
MethodsThe Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines were used to orient the review process. Studies that assessed the impact of physical exercise on children and adolescents undergoing HSCT from database inception were identified. The published protocol can be accessed via the PROSPERO database. An assessment of the quality of evidence was made to support the strength of the findings and conclusions.
Search strategyThe electronic databases Embase, Lilacs, PEDro, PubMed and SCOPUS were searched using the terms: “Exercises” OR “Physical Activity” OR “Activities, Physical” OR “Activity, Physical” OR “Physical Activities” OR “Exercise, Physical” OR “Exercises, Physical” OR “Physical Exercise” OR “Physical Exercises” OR “Acute Exercise” OR “Acute Exercises” OR “Exercise, Acute” OR “Exercises, Acute” OR “Exercise, Isometric” OR “Exercises, Isometric” OR “Isometric Exercises” OR “Isometric Exercise” OR “Exercise, Aerobic” OR “Aerobic Exercise” OR “Aerobic Exercises” OR “Exercises, Aerobic” OR “Exercise Training” OR “Exercise Trainings” OR “Training, Exercise” OR “Trainings, Exercise” AND “Children” OR “Preschool Child” OR “Children, Preschool” OR “Preschool Children” AND “Grafting, Bone Marrow” OR “Bone Marrow Grafting” OR “Transplantation, Bone Marrow” OR “Bone Marrow Cell Transplantation” OR “Transplantation, Bone Marrow Cell” OR “Marrow, Bone” OR “Red Marrow” OR “Marrow, Red” OR “Yellow Marrow” OR “Marrow, Yellow” OR “Marrow” OR “Leukemia” OR “Cancer” AND “randomized controlled trial” OR “controlled clinical trial” OR “randomized controlled trials” OR “random allocation” OR “double-blind method” OR “single-blind method” OR “clinical trial” OR “clinical trials” OR NOT animal[mh] NOT human[mh].
The searches were performed from July to August 2019 by two independent authors. Title and abstract screening were applied to identify relevant articles and remove articles that were not eligible. Based on the satisfaction of the inclusion/exclusion criteria, the article was read in full.
Eligibility criteriaInclusion criteria included randomized controlled trials (RCTs) and non-randomized controlled trials (non-RCTs) including children and adolescents who had undergone bone marrow transplantation and had been part of exercise sessions, including aerobic or resistance training, in the inpatient or post-discharge phases, on whom studies had been written in the English language. The exercise intervention needed to be somewhat structured and described in enough detail to be repeated. A control group received usual care and no additional formal exercise intervention in their usual daily routine. Studies which compared one modality of exercise to another and still met the above criteria were included. No restrictions were applied as to the publication date of the articles, owing to the limited number of studies in a relatively new field.
Exclusion criteria included studies not written in English, providing no original data or that were not peer-reviewed. Studies that were written as abstracts only, rather than full papers, were also excluded.
Risk of bias assessmentThe reviewers independently assessed the risk of bias in the inclusion of studies. The rating of the level of scientific evidence for each identified study was carried out using the Physiotherapy Evidence Database (PEDro) scale. Its criteria evaluate methodological qualities and validity characteristics internally, such as doing the study blindly, using statistics and analyzing with the intent to treat.
Meta-analysis strategyMeasures of treatment effect were performed using the software program Review Manager 5 (RevMan 2014). Continuous data were analyzed using the mean difference (MD), 95% confidence intervals (CIs) being reported for all estimates.
The fixed-effect and random-effect models were applied, depending on the statistic heterogeneity. Moreover, the generic inverse variance method was used to combine RCTs into a single meta-analysis.
As a means to evaluate the heterogeneity of the data, an estimation of the treatment effects reported by individual studies, combined with the heterogeneity between trials, was performed. It included Floret plot analysis and quantification of the heterogeneity impact, using the I² statistic. The degree of heterogeneity was classified as less than 25% (without heterogeneity), 25%–49% (low heterogeneity), 50%–75% (moderate heterogeneity) or greater than 75% (substantial heterogeneity), as recommended by the Cochrane group. When there was evidence of apparent or moderate to substantial heterogeneity (I² greater than 50%), the possible causes were evaluated, searching for evidence of bias or methodological differences among the clinical trials.
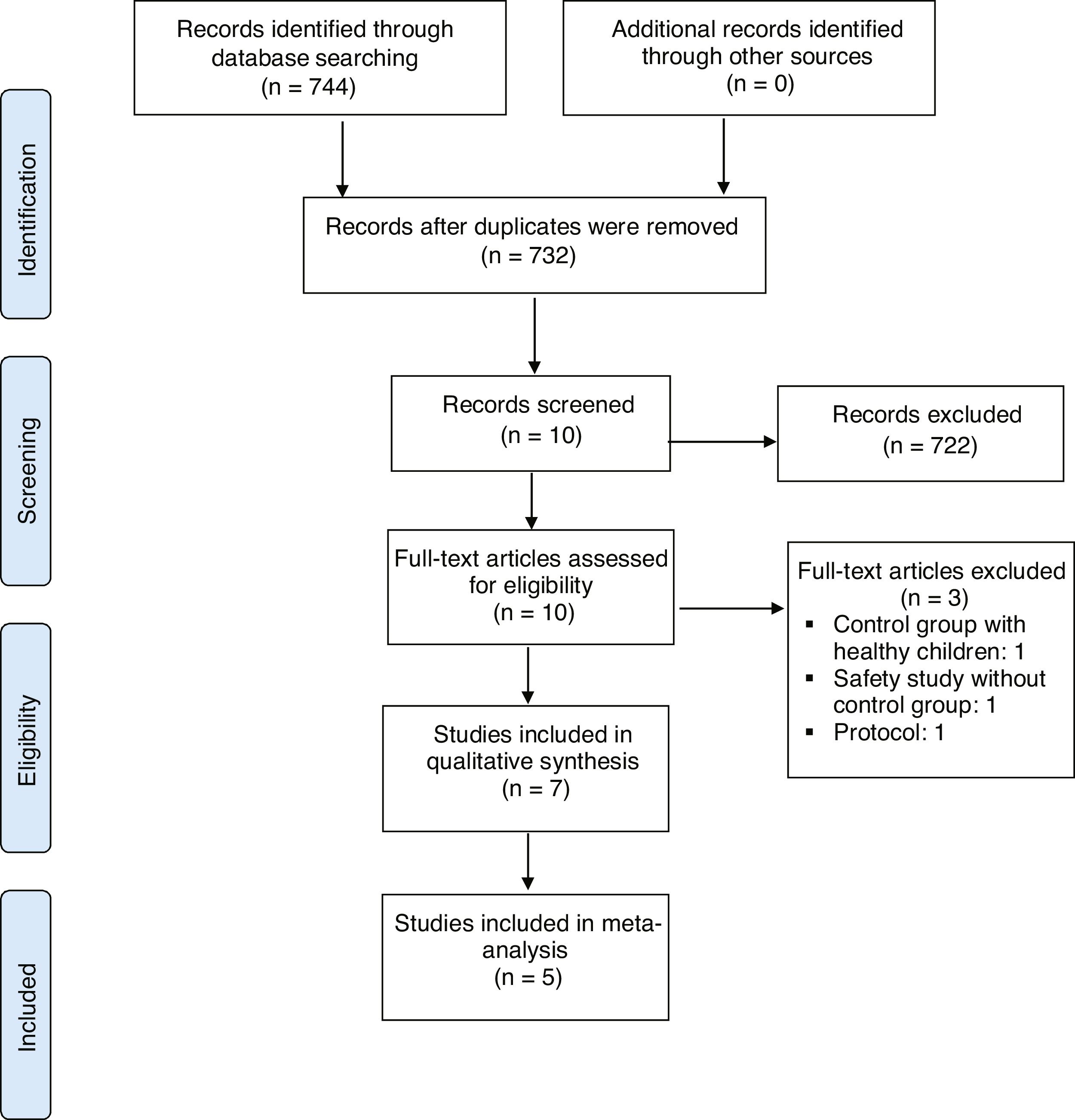
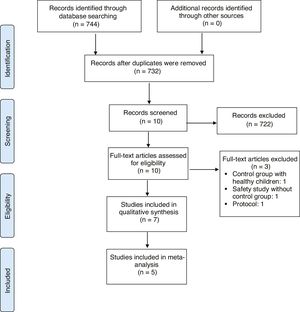
ResultsStudies retrievedStudies retrieved by the details of the results of the literature search are provided in Figure 1. The search criteria resulted in the retrieval of 732 articles, 723 of which were subsequently excluded after their titles and abstracts had been screened according to the eligibility criteria. Full-text screening of the remaining seven studies resulted in two RCTs and five non-RCTs that met all eligibility criteria and were included for review.
Thus, seven RCTs (7 publications) met the eligibility criteria, reporting at least one of the outcomes established for this study. All were parallel studies. In summary, this is the general information on the included studies:
- •
Sample size ranging from 6 to 70 children and adolescents;
- •
Participant age, ranging from 3 to 19 years old, with an average between 8 to 9 years of age.
- •
The studies included both hospitalized and discharged post-HCST patients. Three RCTs described inpatient interventions, three RCTs combined inpatient and home-based exercises and one RCT described only home-based exercises (Table 1).
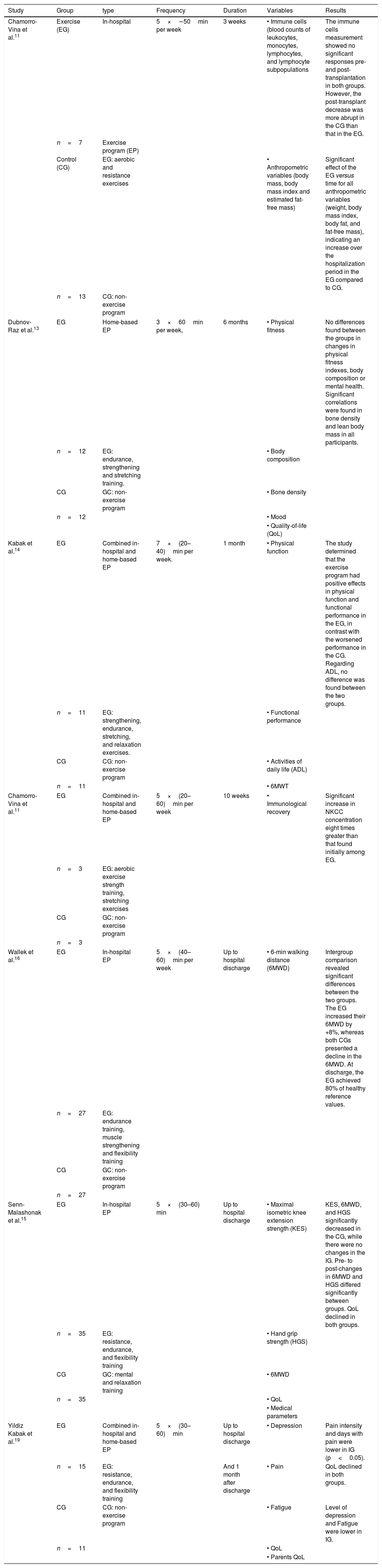
Table 1.Outcomes from exercise intervention.
Study Group type Frequency Duration Variables Results Chamorro-Vina et al.11 Exercise (EG) In-hospital 5×∼50min per week 3 weeks • Immune cells (blood counts of leukocytes, monocytes, lymphocytes, and lymphocyte subpopulations The immune cells measurement showed no significant responses pre- and post-transplantation in both groups. However, the post-transplant decrease was more abrupt in the CG than that in the EG. n=7 Exercise program (EP) Control (CG) EG: aerobic and resistance exercises • Anthropometric variables (body mass, body mass index and estimated fat-free mass) Significant effect of the EG versus time for all anthropometric variables (weight, body mass index, body fat, and fat-free mass), indicating an increase over the hospitalization period in the EG compared to CG. n=13 CG: non-exercise program Dubnov-Raz et al.13 EG Home-based EP 3×60min per week, 6 months • Physical fitness No differences found between the groups in changes in physical fitness indexes, body composition or mental health. Significant correlations were found in bone density and lean body mass in all participants. n=12 EG: endurance, strengthening and stretching training. • Body composition CG GC: non-exercise program • Bone density n=12 • Mood • Quality-of-life (QoL) Kabak et al.14 EG Combined in-hospital and home-based EP 7×(20–40)min per week. 1 month • Physical function The study determined that the exercise program had positive effects in physical function and functional performance in the EG, in contrast with the worsened performance in the CG. Regarding ADL, no difference was found between the two groups. n=11 EG: strengthening, endurance, stretching, and relaxation exercises. • Functional performance CG CG: non-exercise program • Activities of daily life (ADL) n=11 • 6MWT Chamorro-Vina et al.11 EG Combined in-hospital and home-based EP 5×(20–60)min per week 10 weeks • Immunological recovery Significant increase in NKCC concentration eight times greater than that found initially among EG. n=3 EG: aerobic exercise strength training, stretching exercises CG GC: non-exercise program n=3 Wallek et al.16 EG In-hospital EP 5×(40–60)min per week Up to hospital discharge • 6-min walking distance (6MWD) Intergroup comparison revealed significant differences between the two groups. The EG increased their 6MWD by +8%, whereas both CGs presented a decline in the 6MWD. At discharge, the EG achieved 80% of healthy reference values. n=27 EG: endurance training, muscle strengthening and flexibility training CG GC: non-exercise program n=27 Senn-Malashonak et al.15 EG In-hospital EP 5×(30–60) min Up to hospital discharge • Maximal isometric knee extension strength (KES) KES, 6MWD, and HGS significantly decreased in the CG, while there were no changes in the IG. Pre- to post-changes in 6MWD and HGS differed significantly between groups. QoL declined in both groups. n=35 EG: resistance, endurance, and flexibility training • Hand grip strength (HGS) CG GC: mental and relaxation training • 6MWD n=35 • QoL • Medical parameters Yildiz Kabak et al.19 EG Combined in-hospital and home-based EP 5×(30–60)min Up to hospital discharge • Depression Pain intensity and days with pain were lower in IG (p<0.05). n=15 EG: resistance, endurance, and flexibility training And 1 month after discharge • Pain QoL declined in both groups. CG CG: non-exercise program • Fatigue Level of depression and Fatigue were lower in IG. n=11 • QoL • Parents QoL EG: exercise group; CG: control group; EP: exercise program; QoL: quality of life; IG: intervention group; KES: knee extension strength; HGS: hand grip strength; 6MWT: six minute walk test; ADL: activity daily life; NKCC: NK cell cytotoxity.
After full-text reading of the studies alleged for eligibility, three were excluded. San Juan et al.8 included healthy children who were not subjected to HCST in the control group. In addition, the Bogg et al.9 study did not have a control group, even though it had met several outcomes of interest outlined in this systematic review (SR) and meta-analysis. Lastly, the Chamorro-Viña et al.10 protocol was excluded.
Ongoing studiesA search was carried out on the clinicaltrials.org. Two ongoing studies were identified which involve pediatric and adolescent patients undergoing HCST who will take part in a physical exercise program. An Italian study (NCT04090268) started in 2017 aims to enroll 380 patients in a combined exercise protocol for 12 weeks. A French study in its initial phase (NCT04331483) was designed to treat 30 pediatric patients through a supervised EP four times per week for 3 months. The latter is expected to be completed in 2023.
Quantitative analysisAfter establishing similarities between the outcomes assessed in the RCTs, three significant results related to physical exercise in children and adolescents after HCST were selected. These are QoL; physical performance by the distance walked in meters in the 6MWT, as well as the percentage of the predicted distance.
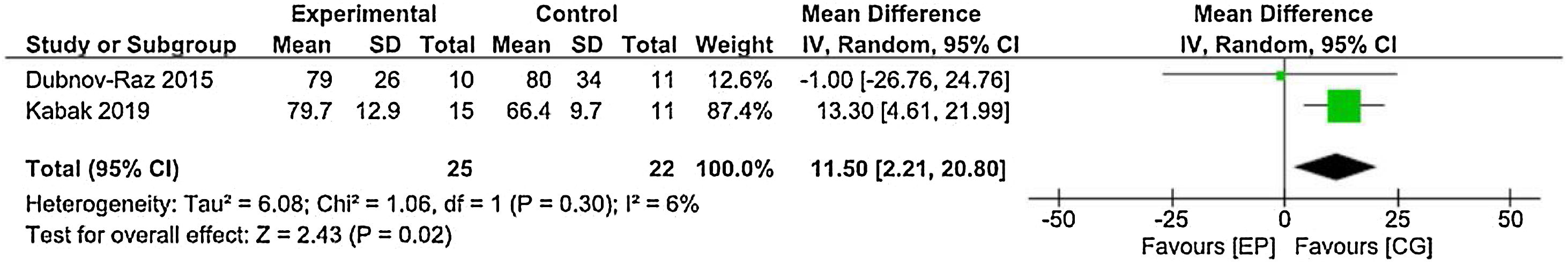
Quality-of-lifeThe Dubnov-Raz et al.13 and Yildiz Kabak et al.19 studies, using the PedsQL to assess QoL, and data from 25 patients in the EG and 22 patients in the CG were included in the statistical analysis. The result represented in the forest plot shows statistical significance, with lower values for QoL in the CG. Physical exercise prevents a reduction in the QoL of these patients (Figure 2).
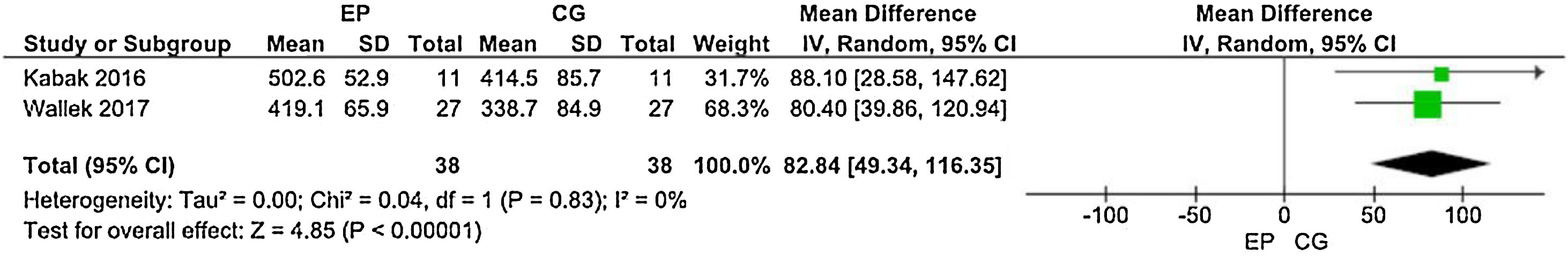
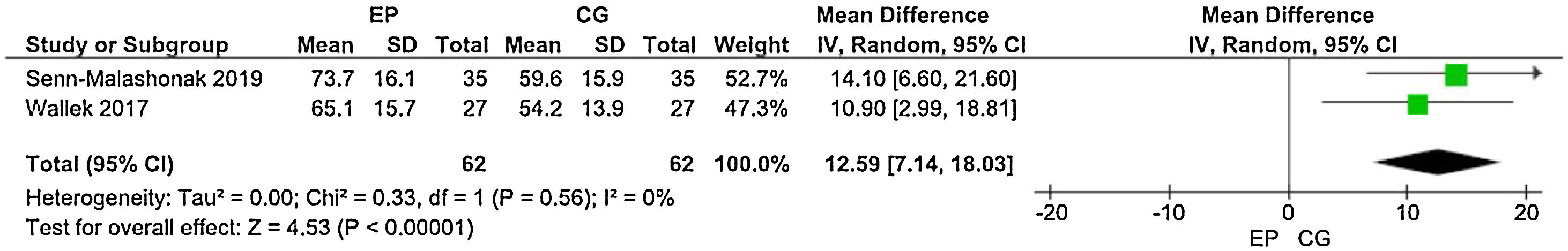
Distance walked in the 6MWTThree studies employed the 6MWT to assess the physical performance of post-HCST children and adolescents. Kabak et al.14 used the distance walked in meters as an outcome measure, whereas Senn-Malashonak et al.15 used the percentage from the 6MWT distance walked, as predicted by formulas. In contrast, Wallek et al.16 assayed the distance walked in meters and the percentage. The forest plots (Figures 3 and 4) present the statistical difference in the shorter distance walked in the CG, both in meters and percentage of the predicted value.
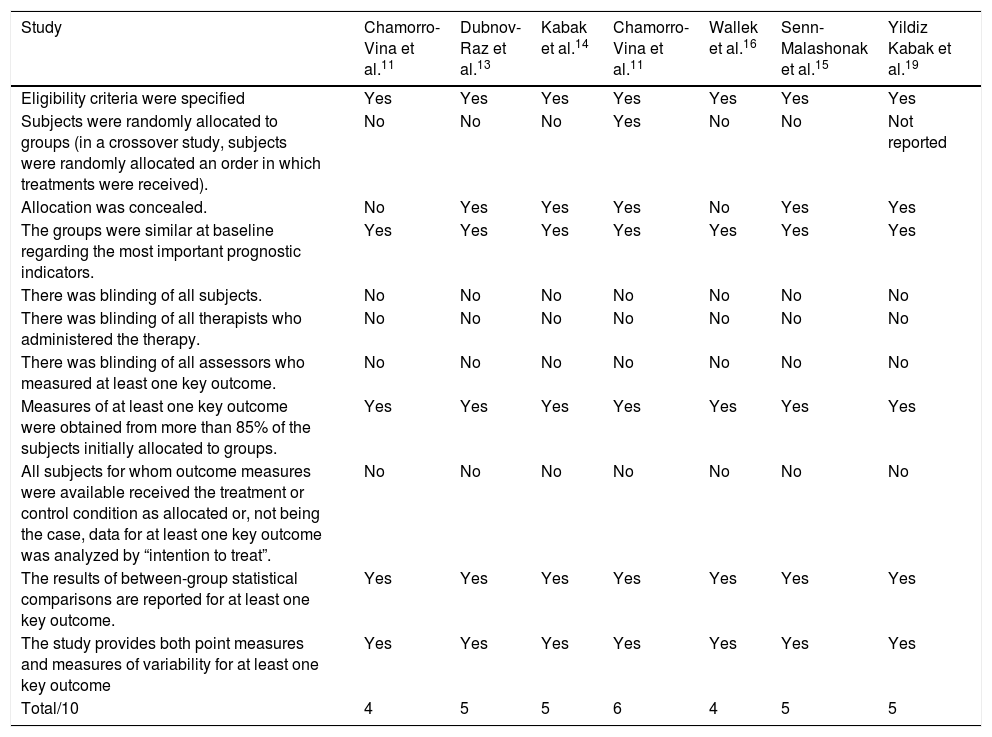
Study quality assessmentThe risk of bias was assessed using the PEDro scale. The methodological quality of the studies lost somewhat in relation to randomization, blinding and the analysis of the intention to treat. However, all of them presented baseline data, an intergroup comparison and a follow-up of at least 85% of the initial sample, indicating little significant sample loss (Table 2).
Study quality assessment — PeDro scale.
| Study | Chamorro-Vina et al.11 | Dubnov-Raz et al.13 | Kabak et al.14 | Chamorro-Vina et al.11 | Wallek et al.16 | Senn-Malashonak et al.15 | Yildiz Kabak et al.19 |
|---|---|---|---|---|---|---|---|
| Eligibility criteria were specified | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Subjects were randomly allocated to groups (in a crossover study, subjects were randomly allocated an order in which treatments were received). | No | No | No | Yes | No | No | Not reported |
| Allocation was concealed. | No | Yes | Yes | Yes | No | Yes | Yes |
| The groups were similar at baseline regarding the most important prognostic indicators. | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| There was blinding of all subjects. | No | No | No | No | No | No | No |
| There was blinding of all therapists who administered the therapy. | No | No | No | No | No | No | No |
| There was blinding of all assessors who measured at least one key outcome. | No | No | No | No | No | No | No |
| Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups. | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| All subjects for whom outcome measures were available received the treatment or control condition as allocated or, not being the case, data for at least one key outcome was analyzed by “intention to treat”. | No | No | No | No | No | No | No |
| The results of between-group statistical comparisons are reported for at least one key outcome. | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| The study provides both point measures and measures of variability for at least one key outcome | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Total/10 | 4 | 5 | 5 | 6 | 4 | 5 | 5 |
Cohorts from the studies show a total of participants, within intervention and control groups, that ranges from 60 to 70 children and adolescents who had undergone HSCT. The studies included a substantial heterogeneity, in terms of historical and patient-specific resources.11–16 The ages of the study groups ranged from 3 to 19 years old.12 Across the total, the mean age ranged from 8 to 9 years old corresponding to the period of childhood, as proposed by this SR.
Three studies, including both aerobic and strength interventions were performed on the inpatient population, while the other three, subsequent to discharge. Frequency of the sessions ranged from 3 to 5 times per week, lasting 20–50min each. The primary outcomes analyzed were the effects of exercise on immunity, physical, functional capacity, bone density, QoL and body composition.
According to the findings, most individuals were post-transplant patients receiving drug administration, including antibiotic prophylaxis.11–13 Information on post-transplant days was reported only in one study, which included individuals up to 30 days post-HSCT.12 It appears essential to have more details on the post-HSCT course to improve and facilitate tailored interventions for the pediatric and adolescent population.
This study purpose was to conduct an SR from the perspective of evaluating the effects of physical activity by post-HSCT pediatric and adolescent patients to synthesize pertinant information about exercise relevance in the motor and respiratory dysfunctions.
Regarding exercise intervention, three studies13–15 showed that even during HSCT, patients may be able to undertake physical activity. The inclusion criteria for the articles in this SR refers only to children and adolescents who underwent the transplant and were allowed to initiate their physical therapy intervention, which does not exclude the possibility of indicating physical activity for patients undergoing other types of hematological treatments.
On the other hand, it is vital to appropriately assess those eligible to commence or not an EP. All of the patients did not tolerate exertion well, and precautions and contraindications were made to promote it without causing harm to them. Orthopedic and neurological diseases, as well as a history of respiratory diseases, were highlighted as exclusion criteria in most studies.
The high acceptance rate and feasibility of an EP for inpatients undergoing HSCT have been described by other authors and it seems to be safe.9 Nevertheless, the studies were divergent regarding the duration, setting (hospital vs. home) and disease severity of the participants.
The clinical trials included the short- and long-term training programs conducted. The results suggest that low-to-moderate intensity exercises five days per week are safe for post-HSCT participants.11,12,14–16 The duration of interventions was 30–40min on average.11–15 The EP were developed under the guidelines for strength and aerobic training in children and adolescents, using nothing beyond the guidelines established.11–16,18
Among the interventions reviewed, three completed intra-hospital programs and three were completed at home. The EP for inpatients is explained individually by a physiotherapist through explicative handouts. The hospital facilities, such as the gymnasium,11,12 gym15 or training centers,14,15 have been mostly used for training purposes in which the patients are monitored closely. The home EP provided at discharge is supervised by patients’ relatives throughout the process. Regular updates made done via phone calls by a rehabilitation practitioner.
In general, the EP can be performed at hospital facilities under the supervision of a physiotherapist or a certified trainer throughout the patient hospitalization11,12,14–16 demonstrating it to be a feasible and accessible option for exercise practice.
Senn-Malashonak et al.15 showed that seven individuals refused to continue due to lack of interest,15 which may, in our view, be due to the choice of the type of intervention applied. Other side effects are not often reported during the EP may be due to a lack of challenging activities or exercise progression. The reasons for non-adherence to treatment with exercise were not assessed in this review and the intensity of the exercises is also not properly described, making it difficult to prescribe the correct progression of exercises for this population.
The structured EP, which were implemented for the intervention groups, consisted of warm-up and cool-down periods,12 dynamic or static stretching exercises,11,12,14 upper limb and lower limb strengthening11,12,15,16 and core muscle exercises,11,12,14 in addition to aerobic.11,12,15–17 Aerobic training for patients in the home program was completed through treadmill use or free running.13 Only one study compared the effects of physical exercise interventions against mental relaxation training in the control group.15
Changes in indices of bone health, muscle mass, muscle strength and functional performance are seen among children and adolescents undergoing anticancer treatments when they have undergone an individualized and well-structured EP, avoiding loss of its benefits even post-HSCT.11,13–16 Moreover, one study suggests that no detrimental effect was seen in moderate-intensity exercise performed early in allogeneic hematopoietic stem cell transplantation, being feasible and possibly improving immune cell reconstitution.12,17
In contrast, Dubnoz-Raz et al.13 presented no significant response to home-based exercise in outcomes, such as physical capacity, body composition, bone indices and mental health in the exercise group, which may be justified by the need for supervision during exercise training and in-person adherence evaluation. The exercise intensity that is relevant to measure the outcomes mentioned is still not evident.
The measuring of the patient exercise/functional capacity is commonly used to determine the severity and functional effects of the disease and the efficacy of therapeutic intervention and to qualify the exercise rehabilitative training intensity, among others. Four studies used a cardiopulmonary exercise test (treadmill or cycle)13,15 and field walking tests (6-min walk test)13–16 to assess the individual's submaximal exercise capacity performed according to The American Thoracic Society. The primary outcome variable was an improvement in the distance walked, mainly in the regular EP group.18
There is a limited number of RCT/non-RCT designed to investigate the outcomes of the EP in children and adolescents who had undergone HSCT. In particular, there are studies comparing exercise modalities, including an exercise combination that designs an appropriate strengthening and aerobic program, in regard to intensity. Although intensity concerns a further element of exercise prescription, none of the studies could establish an appropriate level for HSCT patients.
Moreover, the CRP and musculoskeletal response relation would be of value to assist practitioners in incorporating research findings to provide best practices in home-based and intra-hospital EPs. However, the post-EP long-term follow-up (3–12 months) is still limited, compromising the results found in the intervention studies included in this SR.
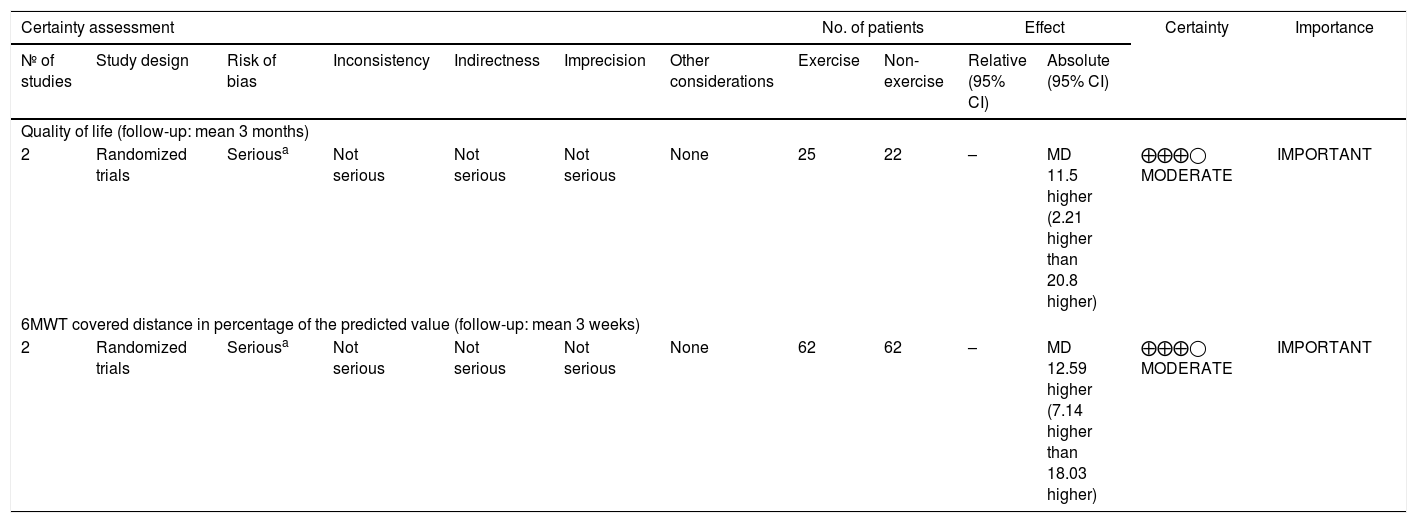
Assessment of evidence qualityThe assessment of evidence quality was carried out using the GRADE approach (Grading Recommendations Assessment, Development, and Evaluation), used to rate the body of evidence at the outcome level, rather than the study level. To determine the strength of the recommendation, GRADE takes into account key factors, such as the risk of bias, inconsistency, indirectness, publication bias and other biases. The quality rating has four levels in certainty in evidence, being very low, low, moderate and high, in which the high rate intervention should be strongly recommended.
Regarding the evidence quality, this SR and MA observed a low certainty in evidence in two outcomes (QoL and 6MWT). Although the studies individually show a moderate methodological quality, due to the small number of patients included in the general sample, the quality of the evidence is low, requiring further studies, as the research question has not yet been answered (Table 3).
Exercise compared to non-exercise for children and adolescents undergoing hematopoietic stem cell transplantation.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Exercise | Non-exercise | Relative (95% CI) | Absolute (95% CI) | ||
| Quality of life (follow-up: mean 3 months) | ||||||||||||
| 2 | Randomized trials | Seriousa | Not serious | Not serious | Not serious | None | 25 | 22 | – | MD 11.5 higher (2.21 higher than 20.8 higher) | ⨁⨁⨁◯ MODERATE | IMPORTANT |
| 6MWT covered distance in percentage of the predicted value (follow-up: mean 3 weeks) | ||||||||||||
| 2 | Randomized trials | Seriousa | Not serious | Not serious | Not serious | None | 62 | 62 | – | MD 12.59 higher (7.14 higher than 18.03 higher) | ⨁⨁⨁◯ MODERATE | IMPORTANT |
CI: confidence interval; MD: mean difference.
This is the best evidence available to suggest that a correctly adapted EP with supervision should include aerobic, strength and flexibility exercises at a frequency of 3–5 times a week since hospitalization and that these also be applied after hospital discharge, being safe, viable and potentially beneficial in terms of cardiopulmonary capacity and musculoskeletal function. In addition to measurements of physiological outcome, it will be valuable in future investigations to include the measurements of immune outcome and QoL in children and adolescents after HSCT.
Conflicts of interestThe authors declare no conflicts of interest.