The new coronavirus SARS-Cov-2, which causes the pneumonia syndrome, named COVID-19, was recognized in China in December 2019 and has now spawned an emergency pandemic condition in the world. The clinical signs and symptoms vary from mild to severe, ranging from pneumonia, fever, fatigue, malaise and cough to severe complications, including cardiac injury, renal failure and life threatening coagulopathy.1,2 Several autoimmune disorders have also been reported in the context of the COVID-19 infection, such as the antiphospholipid syndrome, autoimmune cytopenia, Guillain-Barré syndrome and Kawasaki disease.3 Autoimmune hemolytic anemia (AIHA) was previously described in some viral infections.4 Recently, some reports have shown either cold or warm AIHA in COVID-19 patients.5,6
Herein we discuss a patient with a history of transfusion-dependent thalassemia with multiple alloantibodies, which produced cold agglutinins during the SARS-Cov-2 infection.
Case reportA 49-year-old woman with thalassemia was admitted for blood transfusion due to her low Hb (4.5 g/dL). She had a history of multiple alloantibodies, including anti-c, anti Fyb, anti-JKa and anti-E. The blood specimen was sent to the immunohematology reference lab.
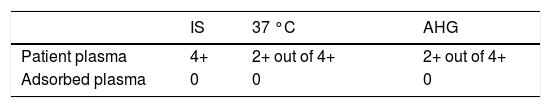
The alloantibodies were reconfirmed with an 11-cell antibody identification panel. The direct antiglobulin test (DAT) using polyspecific anti-human globulin (AHG) was also negative. After 1 week, a phenotype-matched donor was recruited for blood donation; the cross-match test was performed and, unexpectedly, the test was incompatible with 4+, 2+ and 2+ reactions in IS, 37 °C and AHG, respectively. The antibody identification with an auto-control tube and DAT was retested and, surprisingly, the auto-control tube and DAT resulted in positive reactions. The DAT was positive with anti-C3d (4+), but negative with anti-IgG. The cold auto adsorption with rabbit erythrocyte stroma (RESt, Immucor) was performed according to the manufacturer's instructions. Preceding the adsorbing plasma, the cross-matching was examined using phenotype-matched red cells. Based on the patient red blood cell (RBC) phenotype, a phenotype-matched donor was selected for the following antigens: JKa, FYb, E and c. The result revealed negative reactions in IS, 37 °C and AHG (Table 1).
Regarding the above pattern and positive DAT, the presence of a new cold autoantibody was confirmed. Afterward to determine the titer of autoantibody cold agglutinin test was performed which determined as 1024.
While waiting to find compatible blood (one week), the patient developed extremely severe anemia with a decrease in hemoglobin levels from the baseline of 4.5 to 1.5 g/dL (the normal range being 12 to 16 g/dL), elevated indirect bilirubin (2.9 mg/dL, the normal range being 0.2 to 0.9 mg/dL) and MCHC (41 g/dL, the normal range being 33 to 36 g/dL), MCV 131 fL (the normal range being 80 to 96 fL), While the leukocyte count and platelets were normal, the serum lactate dehydrogenase (LDH) was also elevated (355 U/L, the normal range being 125 to 220 U/L). Serology testing for mycoplasma pneumonia (IgM and IgG), as well as laboratory evaluation of anti-cardiolipin (IgM and IgG) and anti-phospholipid antibody (IgM and IgG) were negative. The peripheral blood smear exhibited hypochromic-microcytic erythrocytes with frequent red cell agglutinations, microspherocytes and schystocytes, which corroborate morphologic findings of autoimmune hemolytic anemia (AIHA).
Clinical signs and symptoms of pulmonary infection were noticed, including pneumonia, loss of smell, fever and cough. Chest computed tomography revealed severe interstitial pneumonia. The quantitative RT-PCR result was positive for SARS-CoV-2 from her nasopharyngeal secretions. Therefore, the diagnosis of COVID-19 was confirmed. The interval between admission and the onset of hemolytic anemia was 7 days, which was concurrent with the COVID-19 infection. The treatment for the COVID-19 infection began immediately, but, unfortunately, the patient passed away during hospitalization and no subsequent evaluations were possible.
DiscussionThis report describes a patient presenting a case of thalassemia with SARS-CoV-2- associated with autoimmune hemolytic anemia having cold agglutinins. Secondary CAD has been reported in viral infections, such as the Epstein-Barr virus (EBV), influenza and mycoplasma pneumonia infection.7,8
The underlying mechanism might be antigen mimicry with antigens on the RBC membrane, such as the influenza virus.8 Francesca Angileri et al. recently reported that molecular mimicry between Ankyrin 1 and the viral protein spike is a key factor in AIHA for patients with COVID-19.9 Other than pulmonary complications, which have been reported frequently,1,2 auto immune manifestations, such as AIHA and cold agglutinins, have recently been reported by some authors.5,10,11 Lazarian G et al. reported that the median onset of AIHA in COVID-19 patients was 9 days. Furthermore, 3 out of 7 patients had confirmed cold agglutinins. In addition, 2 cases diagnosed as having B-cell lymphoma and one, prostate cancer.5 The Berzuini et al. study showed Positive DAT in 46% of the patients with COVID-19. The transfusion requirement was significantly higher in the earlier mentioned group than in those with a negative DAT.11 Maslov DV et al. described a patient with COVID-19 who presented with cold autoimmune hemolytic anemia and a high cold agglutinin titer. They also discussed the increased risk of coagulopathy in those with CAD, therefore existing the possibility of more aggressive disease.6
Our patient did not have a history of autoantibody in her serologic tests, so cold AIHA can be attributed to her recent COVID-19 infection. Because the crossmatch was performed and positive results showed positive reactions also in the 37 °C and AHG and then, negative results in the cold auto adsorption test, we did not perform the thermic amplitude test, which constitutes one of the limitations in this study.
ConclusionThe COVID-19 infection might complicate the management of anemia in thalassemia patients, especially those with clinically significant antibodies who need urgent compatible blood. Hence, it is imperative to investigate autoimmune hemolytic anemia in cases with COVID-19.