In serological testing, determination of ABO grouping requires both antigen typing for A and B antigens and screening of serum or plasma for A and B antibodies. Lack of corroboration between the results of the cell and serum groupings identifies a discrepancy. Analysis of ABO blood group discrepancies was performed to determine the incidence of these discrepancies among healthy blood donors and oncology patients.
Materials and methodsABO discrepancies found during testing of blood samples from blood donors and patients in an oncology centre in the period from January 2015 to December 2018 were analysed. ABO blood grouping was performed using the column agglutination test. Detailed serological workups were carried out to resolve discrepancies.
ResultsDuring the study period, a comprehensive analysis was conducted on a large dataset comprising 76,604 blood donor samples and 134,964 patient samples. Of these samples, 117 ABO discrepancies were identified with 13 occurring in blood donor samples and 104 in patient samples. The results demonstrated discrepancies caused by weakened/missing antibodies, weakened/missing antigens, panagglutination and miscellaneous factors in the blood donor samples, with percentages of 0%, 38%, 8%, and 31%, respectively. In patient samples, the percentages were 24%, 27%, 26%, and 15%, respectively.
ConclusionWeakened/missing antigen discrepancies were the prevalent type in both blood donor and patient samples. For accurate blood group reporting and management of transfusion needs of patients, a complete serological workup is vital to resolve any blood group discrepancies.
Blood group antigens are an integral part of the red cell membrane. The presence or absence of certain protein molecules, namely antigens, which are located on the surface of the red blood cells (RBCs), and antibodies, which are present in the blood plasma, determine the differences in human blood. In humans, different types and combinations of these molecules can be found. An individual's blood group depends on genetics. Nobel Laureate Karl Landsteiner discovered that the ABO system contains four major phenotypes, namely A, B, AB, and O, determined by the presence or absence of A and B antigens on RBCs. The presence or absence of naturally occurring antibodies directed against missing A and B antigens also characterizes the ABO system. In serological testing, to determine ABO grouping, typing for both A and B antigens and screening of serum or plasma for anti-A and anti-B are required. Typing for A and B antigens, also known as cell grouping (forward type), involves the use of known commercial antisera sources (anti-A, anti-B) to detect antigens on an individual's RBCs. Testing of serum for antibodies, also known as serum grouping (reverse type), involves the use of known reagents in RBCs, namely A1 and B cells, to detect ABO antibodies in a patient's serum.1
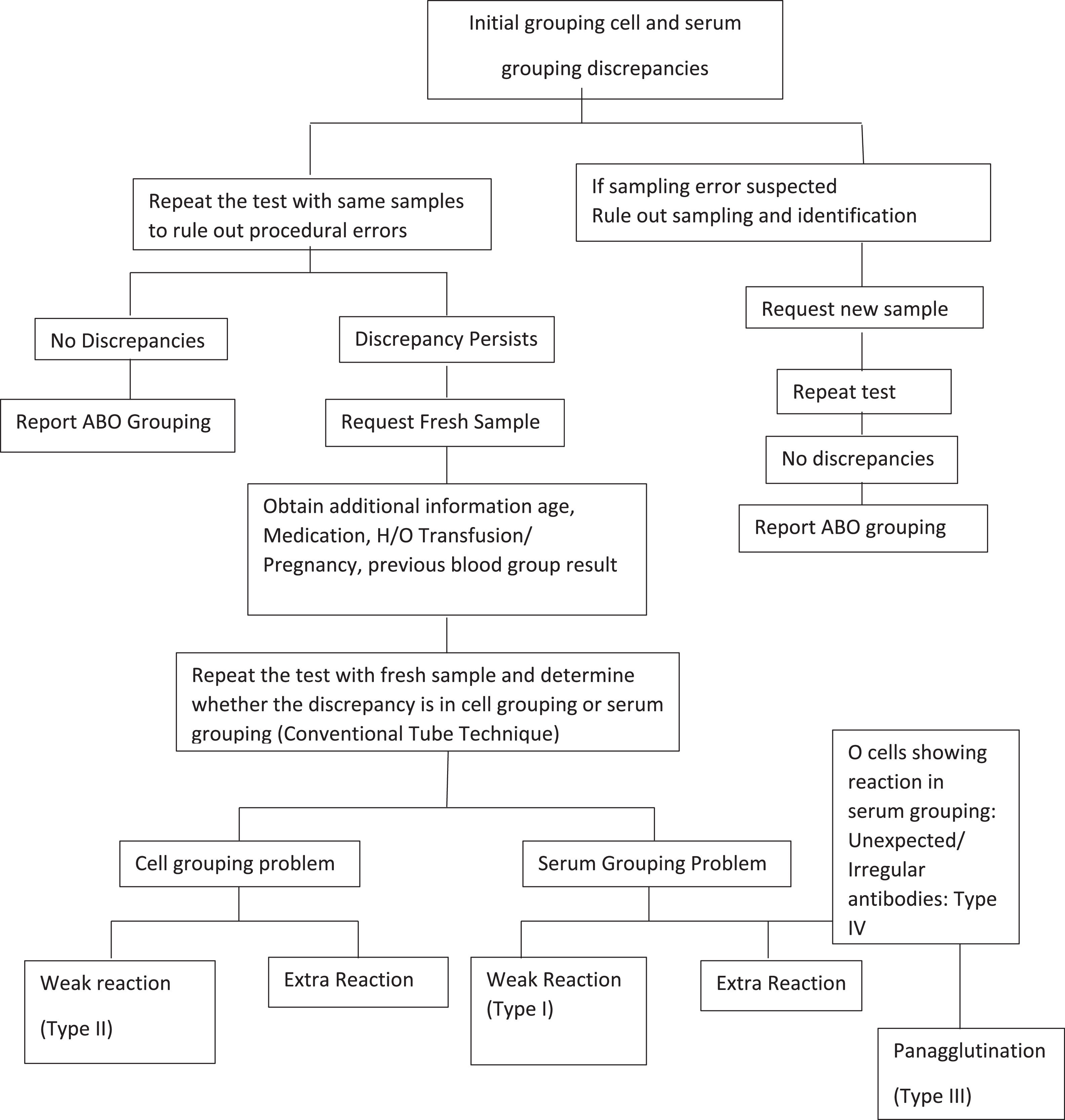
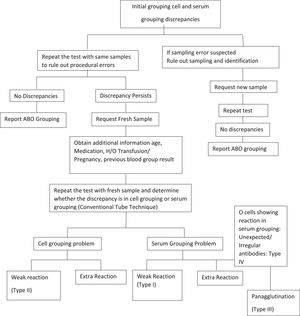
To determine the ABO group of all donor and patient blood samples, performing both ABO cell and serum grouping tests, which serve as checks for one another, is mandatory. Lack of congruence, or unexpected reactions, between the results of cell and serum grouping leads to a discrepancy.2 An unexpected reaction could be the result of an extra-positive reaction or a weak or missing reaction in the cell and serum grouping. Thus, all ABO discrepancies must be resolved before reporting the ABO blood group. A discrepancy may also arise from intrinsic problems with either RBCs or serum or due to technical errors, such as failure to follow standard operating procedures, such as improper centrifugation, improper preparation of cell suspension, missing the reagent addition step, incorrect interpretation of results, or incorrect documentation, while performing the test.3 If discrepancy results persist despite ruling out all causes of technical errors, intrinsic problems of red cell antigens or antibodies in serum/plasma should be considered the main cause. In such cases, a further workup should be planned to resolve the discrepancy.
All discrepancy results must be recorded; however, it is suggested that final interpretation should be postponed until the discrepancy has been resolved. In cases of ABO grouping discrepancies, all the possible technical factors should be reviewed and corrected. If the blood sample is from a donor, the unit should be quarantined and not be released for transfusion. If the blood sample is from a potential transfusion recipient, the transfusion must be stopped or group O–compatible RBCs administered until the discrepancy is resolved. It is vital to record all initial discrepancy results. The following steps explain the process to be followed when an ABO discrepancy is encountered.
- 1.
Repeat the test using the same sample to ensure all technical requirements.
- 2.
Verify reagents and performance of equipment.
- 3.
If discrepancy is not resolved, seek a fresh sample and retest.
- 4.
Review previous records of the blood group test, if available.
- 5.
Review the medical details of the individual from whom the sample was obtained.
- 6.
Perform additional serological testing such as adsorption elution, salivary testing.
ABO discrepancies are divided into the following four groups:1
Type I: In this type, discrepancies are associated with unexpected reactions in the serum (reverse) grouping because of weakened or missing antibodies. This type is more common than the other types of discrepancies.
Type II: In this type, discrepancies are associated with unexpected reactions in the cell (forward) grouping because of weakened or missing antigens. This group of discrepancies is probably the least frequently encountered.
Type III: This type represents discrepancies between cell (forward) and serum (reverse) grouping and is caused by protein or plasma abnormalities and results in rouleaux formation or pseudoagglutination/panagglutination.
Type IV: In this type, discrepancies are caused by miscellaneous issues between cell and serum grouping (due to unexpected/irregular alloantibodies/autoantibodies).
A retrospective study was conducted at a tertiary care oncology centre. Every year, nearly 60,000 blood groups are tested with >20,000 samples from blood donors and >40,000 samples from oncology patients. This study aimed to analyse the incidence, types, and resolution of ABO blood group discrepancies in both donor and patient populations.
Materials and methodsThis was a retrospective observational study to analyse types and resolution of ABO discrepancies in blood donors and patient blood samples in the Department of Transfusion Medicine at a tertiary care oncology centre. Approval from the institutional ethics committee (IEC) to conduct this study was obtained. Blood group results of blood samples from all donors and patients tested during the period from January 2015 to December 2018 were included. Further analysis of all the ABO system discrepancies reported during the study period was carried out.
Testing of blood samplesUsing the departmental standard operating procedure (SOP), the blood samples were tested for ABO and Rh grouping by the column agglutination method on an automated Immunohematology analyser (Autovue Ultra from Ortho Clinical Diagnostics, India). Cell and serum grouping was performed to determine the ABO blood group. All tests were performed as per the manufacturer's instructions. The automated analyser has an inbuilt software that interprets the results of cell and serum grouping for the final blood group report. If the analyser detects a discrepancy, that is, when the cell and serum grouping results do not match, it does not compute the final result. All such samples were retested by using the conventional tube technique for a further workup, as appropriate. Daily quality control checks were done for all the reagents used for both column agglutination and conventional tube testing as per the departmental SOPs, which include the following inclusion and exclusion criteria:
- 1.
Blood samples with ABO discrepancies due to intrinsic problems related to red cell antigens or antibodies were included for analysis.
- 2.
Blood group discrepancies due to technical errors or sampling errors were excluded.
Techniques described in the Technical Manual of the Association for the Advancement of Blood and Biotherapies (AABB) to perform a detailed serological workup in order to resolve observed discrepancies were followed (Figure 1).4
Immunohematology workupIn addition, a fresh blood sample was used in the repeat ABO grouping test by the conventional tube technique to resolve discrepancies. Monoclonal anti-A, anti-B and anti-AB were used for cell grouping and in-house-prepared pooled A cells, B cells and commercially available pooled O red cells (Ortho Clinical Diagnostics, India) were used for serum grouping. Additional reagents like Anti-A1 lectin (Ortho Clinical Diagnostic, India) and Anti-H lectin (purified extract of Ulex europaeus seeds, Tulip Diagnostics) were used whenever required. Extended incubation at 4 °C along with auto control and ‘O’ cells was performed when required. The subgroups were confirmed by using the adsorption and elution techniques. Assessment of saliva for secretor status and the presence of A, B, and H antigens was achieved by means of inhibition tests.5 Human-origin polyclonal antisera from Group B, Group A and Group O individuals were used for the adsorption test to determine these subgroups.6 The heat elution technique was performed at 56 °C for 10 min using 6% bovine serum albumin; the elute was tested against reagent cells (A and B).
Statistical analysisA descriptive analysis of the recorded data, including donor and patient details, such as name, age, gender, medical history, history of transfusion or transplantation, and medication history was performed.
The percentage and mean values were calculated using statistical software. All discrepancies were categorized into four groups, namely, Types I, II, III, and IV, for further analysis.
ResultsA total of 211,568 samples were tested for blood grouping during the study period, of which 76,604 were blood donor samples and 134,964 were patient samples. In total, 117 (0.055%) ABO discrepancies were found of which 13 (0.006%) were found in blood donor samples and 104 (0.049%) in patient samples. Furthermore, 106 (blood donor: 10; patients: 96) of the discrepancy cases were categorized into the four groups, namely Types I, II, III, and IV, for further analysis. The discrepancy in 11 cases (blood donor samples: 3; patient samples: 8) could not be resolved because additional blood and saliva samples were not available for various reasons.
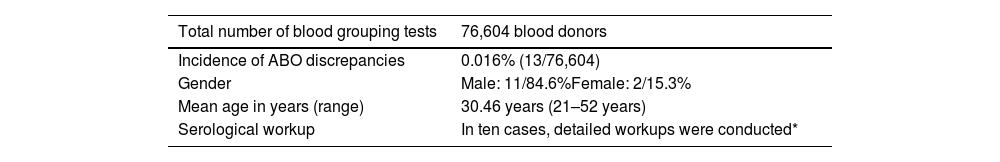
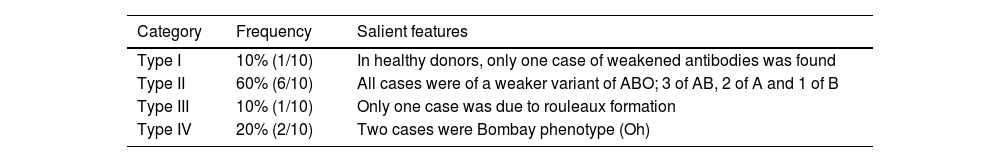
ABO discrepancy in blood donorsOf the 76,604 blood donors tested for ABO blood grouping, 86% (65,839/76,704) were men and 14% (10,765/76,604) were women. Among these donor samples, 13 (0.016%) had ABO discrepancies with a detailed workup being carried out for ten cases. The general characteristics of donors are shown in Table 1. The percentages of Type I, II, III, and IV discrepancies were 10% (1/10), 60% (6/10), 10% (1/10), and 20% (2/10), respectively (Table 2).
ABO discrepancies in blood donors - general characteristics.
| Total number of blood grouping tests | 76,604 blood donors |
|---|---|
| Incidence of ABO discrepancies | 0.016% (13/76,604) |
| Gender | Male: 11/84.6%Female: 2/15.3% |
| Mean age in years (range) | 30.46 years (21–52 years) |
| Serological workup | In ten cases, detailed workups were conducted* |
Categorization of ABO discrepancies in blood donors.
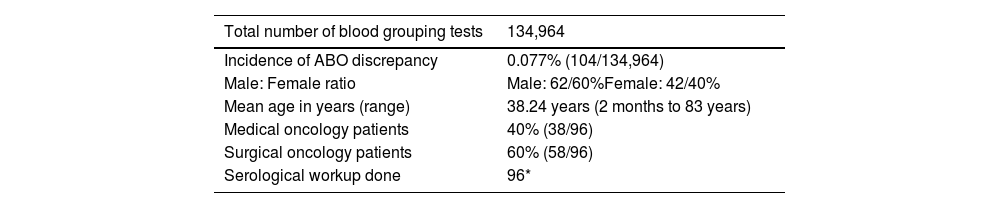
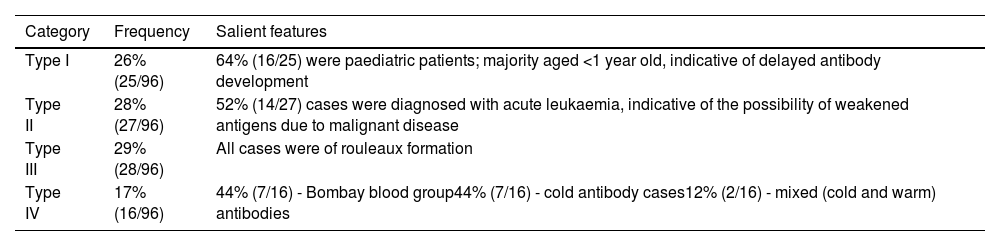
In total, 134,964 oncology patients were tested for blood grouping, of whom 69,641 (51.6%) were men and 65,323 (48.4%) were women. ABO discrepancies were found in 104 patient samples giving an incidence rate of 0.077%. Table 3 shows the general characteristics of patients whose samples proved to have ABO discrepancies. Out of the 104 discrepancy cases, a detailed workup was conducted for 96 and hence, these were considered for the final analysis. Eight cases were excluded. Categorization of these cases indicated that there were 26% (25/96), 28% (27/96), 29% (28/96), and 17% (16/96) of Type I, II, III, and IV discrepancies, respectively (Table 4).
ABO discrepancies in oncology patients - general characteristics.
| Total number of blood grouping tests | 134,964 |
|---|---|
| Incidence of ABO discrepancy | 0.077% (104/134,964) |
| Male: Female ratio | Male: 62/60%Female: 42/40% |
| Mean age in years (range) | 38.24 years (2 months to 83 years) |
| Medical oncology patients | 40% (38/96) |
| Surgical oncology patients | 60% (58/96) |
| Serological workup done | 96* |
Categorization of ABO discrepancies in oncology patients.
Blood grouping is a basic test and establishing standard procedures for blood grouping is essential for blood transfusion services. ABO grouping is the basis for pretransfusion testing; erroneous reporting of ABO grouping or ABO mismatches may result in serious complications. Therefore, accurate reporting of ABO grouping ensures transfusion safety and reduces serious complications due to transfusion of incompatible blood.7-8
The tertiary care oncology centre annually processes more than 50,000 blood samples for blood grouping, including those from blood donors and patients. It is observed that ABO grouping discrepancies are due to technical reasons or intrinsic problems of either red cell antigens or antibodies. The aim was primarily to analyse ABO discrepancies occurring due to intrinsic problems of red cell antigens or antibodies in blood donors and patients visiting the oncology centre. An objective was to assess the incidence, types, and resolution of ABO blood group discrepancies. It was found that the overall ABO discrepancy incidence was 0.055%, with 117 ABO discrepancies reported in 134,964 samples tested during the study period.
ABO discrepancies in blood donorsIn the present study, an incidence of 0.016% of ABO blood group discrepancies was found among blood donors. Similar results have been reported by Sharma et al. (0·04%), Kaur et al. (0·06%) and Makroo et al. (0·02%).8-10
In the present study, it was observed that unexpected reactions in cell grouping due to weakened or missing antigens mainly accounted for ABO grouping discrepancies in blood donors. Sharma et al., in their study about ABO discrepancies in blood donors, reported that weakened or missing antibodies were the most common cause of ABO discrepancies, whereas Kaur et al. found ABO subgroups to be the most common cause.8,9
It was also found that 6/10 (60%) cases of discrepancies were of ABO subgroups, three were of the subgroup AB, two were subgroup A and one was of the subgroup B. There was only one sample with a Type I discrepancy due to a weak antibody reaction; it was resolved by altering the cell-to-serum ratio and extending incubation at 4 °C. One case of Type III discrepancy was caused by the presence of rouleaux formation, which was resolved by the saline replacement technique. There were two cases of Type IV discrepancy, both of which were of the Bombay (Oh) phenotype.
ABO discrepancy in patientsThe incidence of ABO discrepancy in patient samples was found to be 0.077% (104/134,964), which is lower than the incidence of 0.1% reported by Makroo et al.10 Out of 104 discrepancy cases, eight could not be resolved owing to the unavailability of additional samples due to various reasons. Hence, a total of 96 cases were further analysed, of which 26% (25/96), 28% (27/96), 29% (28/96) and 17% (16/96) were categorized as Types I, II, III, and IV discrepancies, respectively. The frequencies of Type II and III discrepancies were equal in our study.
Type I discrepancyOf 25 cases of Type I discrepancies, 64% (16/25) were found in a sample of paediatric patients who were aged <1 year old, indicating the possibility of delayed antibody development. Absent or weakly reacting anti-A and anti-B antibodies can be observed in patients with immunodeficiency, elderly patients, and patients who have recently received bone marrow transplants, as well as in newborns. Anti-A and anti-B antibodies naturally occur and develop from exposure to intestinal bacteria after the age of 4–6 months; hence, serum grouping is usually not conducted for blood samples of newborns. Anti-A and anti-B levels may decrease with ageing because of decreased levels of immunoglobulins. In addition, in conditions such as gammaglobulinemia or hypogammaglobulinemia, anti-A and anti-B may show weak or missing reactions.
Type II discrepancyType II discrepancies were found in 27 cases, in whom cell grouping showed weak or missing antigen reactions. among these 27 cases, 14 (52%) were patients who were diagnosed with acute leukaemia indicating the possibility of weakened antigens due to malignant disease. However, because of the retrospective nature of the present study, it was not possible to confirm the weakly reacting antigens through molecular studies. There have been occasional case reports of changes in ABO blood group antigens in malignant conditions. RBC antigen changes are also occasionally associated with haematological malignancies.11-13 Moreover, ABH antigens have been reported in various malignancies and other hematologic disorders.1 ABH blood group activity in RBCs and other tissues is suppressed due to decreased glycosyltransferase activity involved in the synthesis of blood group substances; therefore, the blood antigen activity is suppressed in RBCs of patients with leukaemia.14
There are two possible mechanisms for the weakening of ABO antigens in hematopoietic diseases: inactivation of A/B transferases and inactivation of the H transferase. In the first mechanism, the expressions of A and B antigens are decreased with a concurrent increase in H antigen expression. The H antigen is not converted to A and B antigens because of inactivation of the A/B transferases. In a study of 12 patients with acute myeloid leukaemia and weakened ABO antigens, it was noted that ABO gene inactivation was not random.15,16
Type III discrepancyAll 28 cases in the Type III category were due to the presence of the rouleaux formation. Esmaili et al., in their study, found 16% of discrepancies were due to the rouleaux formation, which were resolved with the saline replacement technique.17
Type IV discrepancyAmong the 16 cases of Type IV discrepancy, seven were of the Bombay (Oh) phenotype and seven were due to the presence of cold antibodies, whereas two cases were due to the presence of mixed (cold and warm) antibodies.
Individuals with the Bombay blood group with the rare genotype (hh) do not express the H antigen; they are unable to make either A or B antigens on their RBCs, even though they may possess the A, B, or AB blood group genes. The Bombay blood group is prevalent in about one in 10,000 individuals in India.18 In a study from northwestern Orissa, on average, one in 278 individuals with the Bombay phenotype belonged to the Bhuyan tribal population.19 In the present study, it was observed that one of the 19,142 individuals possessed the Bombay phenotype. There is a possibility of incorrectly typing Bombay phenotype samples as the O group if serum grouping does not include testing with O pooled cells.
In seven cases of the current study, the ABO discrepancy was because of the presence of cold antibodies. Makroo et al. reported the presence of cold-reacting autoantibodies as the most common cause of ABO typing discrepancies observed with reverse (serum) typing.10 Arumugam et al. showed that the most common reason for Type IV grouping discrepancies found in their study was because of the presence of alloantibodies followed by cold antibodies and the Bombay phenotype.20 Similarly, Heo et al., in their study, found that cold antibodies were the most common reason for ABO discrepancies.21 Cold agglutinins may create difficulty during routine testing as agglutination is observed with all antisera at room temperature, which can result in the reporting of the sample as AB-positive if serum grouping is omitted. In general, a repeat testing at 37 °C can resolve the problem. Therefore, to avoid the risk of haemolytic transfusion reactions, the treating physician should be informed; transfusions should be given only if absolutely indicated, and clear instructions should be provided to the clinical staff to carefully monitor the patient.
To conclude, Type II was the most prevalent type of ABO discrepancy in this study. Except for 11 cases that could not be resolved, the remaining discrepancies were resolved with an appropriate serological workup, as shown in Figure 1. Completing the serological workup is vital to resolve any blood group discrepancy in order to correctly report the blood group and appropriately manage patient transfusion needs. If ABO discrepancies are found, they should be thoroughly mitigated with available resources, and most of them can be resolved serologically without resorting to advanced investigations.
Molecular methods are useful to confirm serological testing results in ABO discrepancy cases. Whenever possible the blood sample showing an ABO discrepancy should be tested with a molecular technique.