Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal hematological diseases. In addition to defects in hematologic progenitor and stem cells, dysfunctions in the bone marrow microenvironment (BMM) participate in the MDS pathogenesis. Furthermore, the immune response is deregulated by the pro-inflammatory response prevailing in low-risk MDS, while immunosuppression predominates in high-risk MDS. Mesenchymal stromal cells (MSC), part of the BMM, are characterized by plastic adherent growth and multipotentiality. They exhibit immunomodulatory properties and sustain hematopoiesis. There is conflicting evidence regarding their status in MDS. The aim of this study was to characterize MDS-MSC and evaluate the effect of 5-Azacytidine.
MethodsThe MSC from MDS patients and controls were cultured and characterized according to the International Society of Cell Therapy recommendations. Immunomodulatory properties were assessed by studying the MSD cytokine production, using the cytometric bead array. We evaluated the effect of 5-Azacytidine on the MSC cytokine production.
ResultsWe included 35 MDS patients and 22 controls. The MSC from patients and controls were cultured and characterized. The MSC from patients showed morphological differences, but there were no differences in immunophenotype or multipotentiality. The interleukin 6 (IL-6) was the main MSC secreted cytokine. The MDS-MSC produced higher levels of IL-6, IL-17, interferon gamma, or interferon γ (INF-γ), and tumor necrosis factor alpha (TNF-α). The in vitro 5-Azacytidine treatment induced a significant decrease in the IL-6 production by MDS-MSC.
ConclusionsThe MDS-MSC show an increased production of pro-inflammatory cytokines. The in vitro treatment with 5-Azacytidine lead to a significant reduction in the IL-6 production by the MDS-MSC, restoring the IL-6 levels to those found in controls. The MSC produced inflammatory cytokines involved in the MDS pathogenesis, representing a potential future therapeutic target. Moreover, 5-Azacytidine may have a stromal effect, modulating the immune response in MDS.
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal hematological disorders characterized by peripheral blood cytopenia and dysplasia in one or more myeloid linages.1 They share a variable risk of transformation into acute myeloid leukemia (AML). The MDS mostly affect patients over 50 years old. The prevalence is 1/500 in people over 60 years and constitutes the most frequent malignant hematologic disease in this population.
The MDS pathogenesis is characterized by clonal proliferation of hematopoietic stem and progenitor cells (HSPC), which retain differentiation capability, but in an abnormal and ineffective manner. One of the mechanisms underlying this phenomenon is an increased HSPC apoptosis that accompanies the increased proliferation rates.2 Balance between these two phenomena varies within risk groups, with more apoptosis in the low-risk setting, whereas proliferation is the hallmark of high-risk MDS.
However, there is accumulating evidence that implies that the pathogenesis of MDS depends not only on HSPC alterations, but also is supported by the bone marrow microenvironment (BMM), specifically by mesenchymal stromal cells (MSC). In physiological conditions, the MSC play an important role in supporting hematopoiesis and regulating the HSPC proliferation and differentiation.3 In MDS, the BMM is modified by cytokines and soluble factors produced by the neoplastic clone. Moreover, an altered BMM enables MDS clone growth and supports altered hematopoiesis above normal hematopoiesis. The result is a dysfunctional BMM that promotes the disease progression.4 Evidence from animal models showed that an impaired BMM is sufficient to induce a dysplastic hematopoiesis.5 Moreover, the engraftment of human HSPC is dependent on the co-administration of MSC,6 highlighting the importance of the BMM in the MDS pathogenesis.
There has been contradictory evidence regarding the MSC alterations in MDS and their contribution to the progression of AML. Although there is sufficient agreement in morphologic differences and growth impairment,7 there are controversial data regarding the immunophenotype, multipotentiality and immunomodulatory properties.8,9
The 5-Azacytidine is a hypometilating agent (DNMT inhibitor) which is widely used in MDS treatment. It is the current standard of care for patients with high-risk MDS and has also shown activity in lower-risk patients.10,11 Although the mechanism of action is not exactly known, it is proposed that the epigenetic regulation of the HSPC favors the elimination of tumor cells, as well as enhances normal hematopoiesis. The direct effect of 5-Azacytidine on the ability to sustain hematopoiesis by the MSC in MDS patients has been recently published by two different groups.12,13 Moreover, the effect of azacytidine on the gene expression of the MDS-MSC has been demonstrated to affect genes that are actively involved in the support of hematopoiesis.14
In this study, we investigate whether the MSC from patients with MDS have different morphology, immunophenotype, proliferation rate, multipotentiality and immunomodulatory properties, compared to control MSC. Additionally, we examined the effect of the in vitro 5-azacytidine treatment.
Materials and methodsPatients and controlsWe conducted a transversal study at the MDS Unit at the Clinicas Hospital Dr. Manuel Quintela, Montevideo, Uruguay. The inclusion criteria included patients over 18 years old, with untreated MDS. Patients who did not need an additional bone marrow (BM) aspirate were excluded, so that no unnecessary studies would be performed. All patients were risk-classified according to the 2008 World Health Organization (WHO) classification and the International Prognostic Scoring System (IPSS).1,15
The control group was comprised of patients without hematologic disorders nor immune or infectious diseases who were enrolled in the cell therapy clinical trials at our Cell Therapy Unit (patients with venous ulcers and patients with open bone fractures) or patients who underwent cardiac surgery at our institution.
The BM samples from the MDS patients and controls were collected by aspiration in the sternum or posterior iliac spine.
The research protocol was approved by the Institutional Ethical Committee at our Hospital and was conducted in accordance with the Declaration of Helsinki. Both patients and control subjects gave signed informed consent before participating.
MSC: MSC isolation and cultureThe MSC were isolated from BM samples. Mononuclear cells were separated by gradient centrifugation, using the Ficoll Hystopaque (Sigma-Aldrich, St. Louis, MO), and then cultured, using the Dulbecco's Modified Eagle Medium (DMEM) Low glucose (Gibco-BRL, USA), supplemented with 20% of fetal bovine serum (FBS; Thermo Fisher Scientific), 2 mM L-glutamine (Sigma) and antibiotics (penicillin and streptomycin, Sigma), at a seeding density of 300,000 cells/cm2. The cells were cultured at 37 °C in a 5% CO2 atmosphere. After 5 days, the medium was changed in order to remove the non-adherent cell fraction and adherent cells were cultured until confluence was achieved (first passage). In sequence, subsequent passages were performed, using a concentration of 2 × 104 cells/cm2. Morphologic and immunophenotypic characterization, differentiation and cytokine production assays were performed, using the MSC from passages 3 to 6.
Characterization of MSCThe morphologic characterization included microscope observation, using a Ti-S Nikon microscope. The cells were observed in culture at 4×, 10× and 40× and photographs were taken, using the NIS Elements D software (Nikon, EEUU). In order to compare the MSC proliferation capacity, isolated cells from patients and controls were plated and cultured, using the same conditions until confluence, and the time to reach the first passage was measured.
The cultured MSC from MDS patients (MDS-MSC) and controls (control-MSC) were detached enzymatically, using 0.25% trypsin, and resuspended in phosphate-buffered saline (PBS). The cells were immunostained with the following panel of antibodies: PECy5-conjugated CD90, PE-conjugated CD73, PE-conjugated CD105, PerCP-conjugated CD14, PerCP-Cy5.5-conjugated CD34 and FITC-conjugated CD45 (all reagents from BD Pharmingen, San Diego, USA). Optimal antibody concentrations were previously defined by titration. A total of 1 × 105 cells were incubated with antibodies, previously described for 30 min in the dark at room temperature. Then cells were washed and resuspended in PBS and analyzed. The flow cytometry data was collected on a FACS Calibur flow cytometer equipped with two lasers (Becton–Dickinson, Oxford, UK). At least 10,000 events were acquired. The CellQuest software (Becton–Dickinson) was used for the data acquisition and the Infinicyt (Cytognos, Spain), for data analysis.
The differentiation assays were performed with a specific kit (R&D, USA), following the manufacturer instructions. The MSC were cultured using specific supplemented mediums to induce osteogenesis, adipogenesis and chondrogenesis, respectively. Differentiation was assessed by the specific tissue stains Oil red for adipocytes, Alizarin red for osteocytes and Toluidine Blue for chondrocytes.
MSC cytokine production and 5-Azacytidine effectThe MDS-MSC and control-MSC were seeded in duplicates into 24-well plates (Nunc, Naperville, IL), at a concentration of 30,000 cells/cm2 with 2 mL of cultured medium (DMEM low glucose supplemented with 20% of FBS, 2 mM L-glutamine and antibiotics). The cells were treated with one of the following: 5-Azacytidine 1 µM, 5-Azacytidine 5µM or left without treatment as controls. After 96 h of culture, the supernatant was removed and cryopreserved at −80 °C for further analysis. The MSC were detached with trypsine and then the cell viability was assessed by flow cytometry, using iodine propidium.
The in vitro MSC production of cytokines was evaluated in the collected supernatants. The cytokines were measured, using a cytometric bead array (CBA) for human Th1/Th2/Th17 cytokines (Human Th1/Th2/Th17 Cytokine Kit, BD Biosciences, USA), following the manufacturer instructions. We measured the IL-2, IL-4, IL-6, IL-10, INF-γ, TNF-α and IL-17A. The data were acquired with a FACS Canto II cytometer, using the digital instrument CBA template (BD) for FACS DIVA. The data were analyzed by the FCAP Array software (BD).
Statistical analysisThe categorical variables were expressed as frequencies and percentages and quantitative variables, as mean, median and standard deviation or interquartile range (IQR). Due to the sample size, we used non-parametric tests to compare variables between and within groups. We used the Mann Whitney test to compare the patients and controls and the Wilcoxon test to assess the 5-Azacytidine effect within the groups. We considered significant an alpha error under 5%. The analysis was performed with the Statistical Package for the Social Sciences (SPSS) 17.0 (IBM, Spain).
ResultsMDS patients and control subject demographyWe included 35 untreated MDS patients and 22 controls. The median age was 68.5 (30–77) years for the controls and 71.0 (26–90) years for the MDS patients. Our study population was represented mainly by low-risk patients (low and intermediate 1, according to the IPSS), 11.4% were treatment-related MDS and 5.7% were hypoplastic MDS (Table 1).
Patient characteristics.
| MDS patients | |
|---|---|
| n = 35 (%) | |
| Age Median (IQR) | 71 (26–90) |
| Sex (M:F) | 0.7:1 |
| FAB classification | |
| – RA | 25 (71.4%) |
| – RARS | 1 (2.9%) |
| – RAEB | 5 (14.3%) |
| – CMML | 4 (11.4%) |
| WHO classification (2008) | |
| – RCUD | 5 (15.6%) |
| – RCMD | 21 (59.4%) |
| – RARS | 1 (3.1%) |
| – RAEB1 | 1 (3.1%) |
| – RABE2 | 5 (15.6%) |
| – 5q- | 1 (3.1%) |
| IPSS | |
| – Low risk | 18 (50.0%) |
| – Intermediate 1 | 12 (34.4%) |
| – Intermediate 2 | 5 (15.6%) |
| – High risk | 0 |
Data shown are absolute frequency and percentage.
IQR: interquartile range; RA: refractory anemia; RARS: refractory anemia with ring sideroblasts; RAEB: refractory anemia with excess blast; CMML: chronic myelomonocytic leukemia; RCUD: refractory cytopenia with unilineage dysplasia; RCMD: refractory cytopenia with multilineage dysplasia.
We studied 35 BM samples from the MDS patients and 22 from controls in order to evaluate cell culture and expansion. We found that the control-MSC were able to grow in culture and reach the first passage in 18 (81.8%) of the evaluated samples, while in the MDS-MSC, only in 22 (62.8%) of the cases (p was not significant (NS)) did so. The lack of proliferation of the MSC was the main reason why some samples did not reach the first passage. Additionally, we evaluated the time required to reach the first passage, which was 29.7 ± 16 days for the MDS-MSC and 23.8 ± 6.5 days for the control-MSC (p was NS).
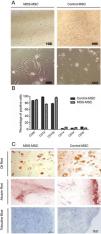
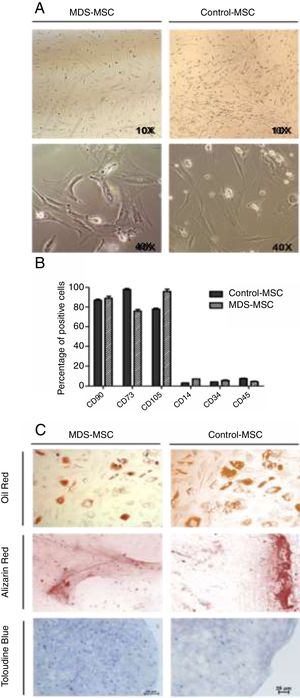
We also analyzed the morphologic characteristics of the MSC in patients and controls. While control-MSC showed fibroblastoid appearance with many ramifications, the MDS-MSC were larger and exhibited fewer ramifications (Fig. 1A). The in vitro 5-azacytidine treatment changed the MSC morphology and the cells exhibited a rounded shape, with less and shorter ramifications after treatment.
MSC characterization. A) Representative light microscopy images showing MSC morphologic differences between MDS patient (right) and control (left) with ×10 and ×40 magnification. B) Flow cytometry analysis of MDS-MSC (n = 5) and control-MSC (n = 5) immunophenotype. Histograms show percentage of positive cells for CD90, CD73, CD105, CD14, CD34 and CD45 (p was not significant). C) Differentiation assay. Representative images of MDS (right column) and control (left column) differentiation assays showing adipogenesis stained with Oil Red, osteogenesis stained with Alizarin Red and chondrogenesis stained with Toloudine Blue.
The immunophenotype was assessed by flow cytometry, as described in the materials and methods section. The MDS-MSC and control-MSC showed the typical immunophenotype of the MSC described by the International Society for Cellular Therapy (ISCT).18 Ten immunophenotyped assays were performed, n = 5 in MDS-MSC and n = 5 in control-MSC. The cells expressed CD73, CD90 and CD105 and were negative for CD45, CD34 and CD14 (Fig. 1B). No differences were found between patient and control immunophenotypes.
In order to evaluate the multipotentiality of the MSC, 17 differentiation assays to osteogenic, adipogenic and chondrogenic linage were performed (Fig. 1C). Ten assays were made for the control-MSC and 7 for the MDS-MSC. Differentiation occurred in 7 of the 10 control-MSC assays (70%) and in 4 of the 7 MDS-MSC assays (57%). There were no differences (p was NS) between the MDS-MSC and control-MSC, regarding the differentiation capacity.
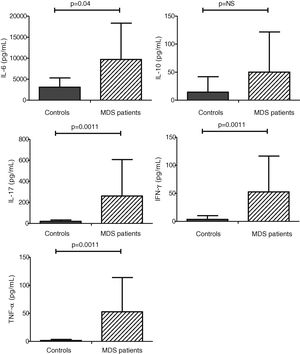
MDS-MSC produced higher amounts of cytokines than controlsCytokine levels in culture supernatant were measured by the CBA assay. We performed a total of 17 CBA assays. For cytokine production, we studied 10 MDS patients and 7 controls. We identified IL-6 as the main MSC-secreted cytokine in both the MDS-MSC (9684.6 ± 8634.4 pg/ml) and control-MSC (3114.8 ± 2182.4 pg/ml). As shown in Fig. 2, the MDS-MSC produced higher amounts of IL-6, IL-17A, INF-γ and TNF-α than the control-MSC. When we evaluated cytokine concentration by risk group, there was a trend toward higher levels in low-risk patients 10,948.2 ± 9231.7 pg/ml compared to high-risk patients 4630.3 ± 3248.5 pg/ml, however, the difference was not significant.
Treatment with azacytidine showed no cytotoxic effect on MSCViability assessment by flow cytometry, using Propidium Iodide in 5 patients and 5 controls showed no significant differences between MSCs treated with Azacytidine or left without treatment. The viability rate was 90 and 92%, respectively (p was NS).
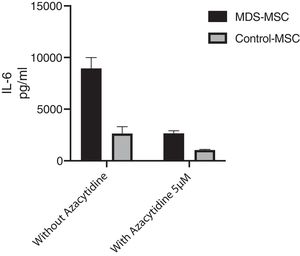
Azacytidine restores MDS-MSC IL-6 productionWe studied the in vitro effect of 5-Azacytidine on MSC cytokine production. We studied 10 MDS patients and 7 controls. The in vitro treatment with two different doses of 5-Azacytidine (1 μM and 5 μM) induced morphological changes in cultured MSC and a significant decrease in the MDS-MSC IL-6 production. While the IL-6 level in the culture supernatant from the MDS-MSC without 5-Azacytidine was 9684.59 ± 8191.36 pg/ml, 7388.18 ± 8224.82 pg/ml after 5-Azacytidine 1 μM (p = 0.017), and 2475.12 ± 2828.32 pg/ml after 5-Azacytidine 5 μM (p = 0.002), the 5-azacytidine treatment in the control-MSC slightly decreased the IL-6 production; however, this effect was not significant. The IL-6 level in the culture supernatant from the control-MSC without 5-Azacytidine was 3114.79 ± 2182.35 pg/ml, 1886.37 ± 1099.72 pg/ml after 5-Azacytidine 1 μM (p = NS) and 1002.26 ± 1086.81 pg/ml after 5-Azacytidine 5 μM (p was NS). Moreover, the IL-6 levels in the MDS-MSC culture supernatant after 5-Azacytidine treatment were not statistically different from the basal IL-6 production by the control-MSC (p = 0.417), suggesting that 5-azacytidine restores the IL-6 production in the MDS-MSC cells (Fig. 3).
In vitro IL-6 levels produced by cultured MDS-MSC and control-MSC treated for 96 h with 5 μM of 5-azacytidine and without treatment as control. IL-6 levels were measure by cytometric bead array (CBA). These results correspond to the 17 assays that were performed (10 samples from MDS-MSC and 7 samples from control-MSC). Data shown are mean and standard deviation (SD).
There was not a significant difference in the levels of IL-2, IL-4, IL-10, IFN-γ, TNF-α and IL-17A produced by the MSC with 5-azacytidine treatment in patients, nor in controls (Data not shown).
DiscussionRecently, there has been a very important breakthrough in what we know about the role of the BMM in the MDS pathogenesis.2,17 Ineffective hematopoiesis is driven not only by alterations in the MDS clone (HPSC), but also in the BMM.17
The MSC are a main component of the BMM and the evidence regarding their characteristics in the MDS are contradictory.9,18 Whereas some groups reported differential immunophenotype19 and impaired osteogenic differentiation,20 others argue that these characteristics are not altered.18 Regarding immunomodulatory properties and hematopoiesis support, there is similarly no consensus.8,18
In this study, we characterized and compared morphology, immunophenotype, and multipotentiality from cultured MSC from MDS patients and control subjects. We were able to expand the MDS-MSC and control-MSC, according to the ISCT criteria.16 In concordance with previous reports, we found morphological differences in the MDS-MSC,7,12,19 with larger size and fewer ramifications than in the control-MSC. We found a trend towards more difficulties in expanding the MDS-MSC than the control-MSC, as a lower proportion of samples reached the first passage and we observed a tendency toward more days till passage 1. While many authors agree that this arduousness in culture is a hallmark19 of the MDS-MSC, recent publications showed no difficulties in the culturing process.21
We found no differences in immunophenotype and multipotentiality capacity between the MDS-MSC and control-MSC. Corradi et al. have published similar results, showing that the MDS-MSC expressed typical MSC markers, without differences in the control-MSC. Recent reports showed differences in the MDS-MSC adhesion molecules expression, such as the CD49b, CD49d and CD49e.14 We only used the ISCT criteria to characterize the MSC and we did not include additional markers, such as adhesion molecules by flow cytometry19 or protein expression in differenced cells (adipocytes, osteocytes and chondrocytes) by immunohistochemistry to see differences in the MSC between controls and MDS patients. Fei et al. have shown differences in the MDS-MSC osteogenic differentiation.20 These discrepancies with our results may be due to different methodologies used to study and to induce differentiation of the MSC. For example, one difference is the use of immunohistochemistry to asses tissue-specific protein expression. Additionally, we believe that another factor in the morphology, immunophenotype and multipotentiality differences may be the diverse representation of the risk groups among the MDS patients. In a disease that constitutes a large and heterogeneous group of different entities, with differences in pathogenic features between risk groups,2 the proportion of each group in the population studied may influence results and make them incomparable. In our study, the population consisted mainly of low-risk MDS patients (low and Intermediate 1, according to the IPSS), thus our results may reflect the status of the MSC in this risk group of MDS patients.
Many studies have shown that the MSC have immunoregulatory properties. They have the ability to boost the suppression of T-cell activation and proliferation in vitro, modify T-cell cytokine production, which lead to a disbalance in TH1/TH2, suppress B and NK cells function and increase the suppressive capacity of regulatory T-cells.22 It has been postulated that the MSC regulatory mechanisms are mediated by soluble factors and cell-cell contact.23 Moreover, there is growing evidence demonstrating the presence of an inflammatory BMM in the MDS. Thus, we decided to test the in vitro Inflammatory/TH1/TH2/TH17 cytokine production by the MDS and control-MSC.
Our results showed that IL-6 was the main in vitro-produced MSC cytokine. The IL-6 is a pro-inflammatory cytokine participating in the MDS pathogenesis as an apoptosis inducer. The IL-6 MSC production has been previously reported.18 In our study, we found that the IL-6 levels were significantly higher in the supernatant from the MDS-MSC compared to the control-MSC. Although IL-6 is a well-known cytokine in the MDS involved in the low-risk MDS pathogenesis2 and increased plasmatic levels have been reported as a negative prognostic factor,24 there were no previous reports of in vitro-increased IL-6 production by the MDS-MSC. Chen et al. have reported an increased mRNA expression of IL-6 in fresh non-expanded MDS-MSC from low-risk patients.25
We also observed a higher production of other inflammatory cytokines (IL-17A, IFN-γ and TNF-α) by the MDS-MSC. Both the IFN-γ and TNF-α are inflammatory cytokines that have been involved in the MDS pathogenesis.26 They increase the FAS receptor expression in the HSPC, making them more prone to FAS ligand induced apoptosis. Some authors reported an increased expression in the bone marrow of MDS patients and also increased gene expression of the MDS-MSC. However, to our knowledge, there has been no previous report of increased IFN-γ and TNF-α in vitro production by the MDS-MSC. The IL-17A is another pro-inflammatory cytokine involved in many immune-mediated diseases. Although the production of IL-17 bone marrow mononuclear cells has been reported to be increased in MDS patients,27 there has been no previous report of their production by the MDS-MSC. Our findings allow us to hypothesize that the IL-6, IL-17A, IFN-γ and TNF-α produced by the MSC may participate in the HSPC deregulation, leading to ineffective hematopoiesis. The possibility of modulating the MSC cytokine production could be a potential therapeutic strategy.
The 5-Azacytidine is a widely used drug in the treatment of MDS. Although the mechanism of action is thought to depend on epigenetic modulation of the HSCP, little has been studied about the effect of 5-Azacytidine on the BMM. Recently, Wenk et al. and Poon et al. showed that 5-Azacytidine is able to modulate the BMM and specifically, the MSC.12,13 Moreover, Bhagat et al. have recently published that untreated MDS-MSC show an aberrant hypermethylation pattern that is not present in the patients treated with 5-azacytidine.28
We found that in vitro 5-Azacytidine is able to modulate the IL-6 production by the MDS-MSC and restore it to levels similar to those found in the control-MSC. This was not due to a higher cytotoxicity in 5-Azacytidine-treated cells, as the viability test with propidium iodide did not show a decreased viability in treated cells. However, we cannot rule out a cytostatic effect. Moreover, the 5-Azacytidine treatment did not modulate the IFN-γ, TNF-α and IL-17A production (data not shown), suggesting that the effect on the IL-6 production is due to a direct effect on the MSC. Similarly to our results, Wenk et al. have reported that viability was not affected in either controls or MDS-MSC after treatment with 10 µM of 5-Azacytidine.12
Regarding the mechanism through which the 5-Azacytidine influences the IL-6 production, there is evidence in other cell types, such as macrophages, supporting the 5-Azacytidine modulation of the LXRα and PPARY1, two nuclear transcription factors leading to a down-regulation of the IL-6 production.29 These nuclear transcription factors participate in the MSC transcriptional regulation.30
In conclusion, we found significant differences in the immunomodulatory properties of low-risk MDS-MSC. There is an increased production of inflammatory cytokines involved in the low-risk MDS pathogenesis. We postulate that the MSC, as part of the BMM, are one of the sources of these cytokines and the effect of 5-Azacytidine on the IL-6 production could have a role in the MSC modulation and could be a potential therapeutic target for the MDS.
Conflicts of interestThe authors declare no conflicts of interest.
MB earned her Magister degree through the Programa Nacional de Investigaciones Biomédicas (ProInBio) and received a postgraduate grant from the Agencia Nacional de Investigación e Innovación (ANII).