Myelodysplastic syndromes (MDS) are an heterogeneous group of clonal hematopoietic disorders characterized by dysplasia, bone marrow (BM) failure and an increased risk of acute myeloid leukemia (AML).1
Human immunodeficiency virus (HIV) infection is a chronic disease requiring lifelong treatment with combination antiretroviral therapy (ART). Thanks to ART, HIV infected patients live longer, with life expectancies similar to non-HIV patients. However, patients with well controlled HIV infection have increased rates of non-AIDS associated diseases such as cancer, neurocognitive impairment and cardiovascular disease (CVD).2 Chronic immune activation in HIV patients on ART, secondary viral co-infection as well as lifestyle factors are thought to be responsible for increased cancer burden in this population. Although people living with HIV are at increased risk of hematological malignancies (non-Hodgkin and Hodgkin lymphoma), the risk of MDS is not well established.2 Less than 50 cases of MDS in HIV patients have been reported in literature as case reports or case series.3 Therefore, management strategies are widely undefined.
We present 2 patients with MDS in the setting of well controlled HIV infection. We discuss diagnostic challenges, treatment with azacytidine and lenalidomide in this setting and the pathophysiology of HIV in MDS development.
Case 1A 78-year-old Caucasian man with 16 years HIV infection, well-controlled on ART (tenofovir, lamivudine, efavirenz), was referred to our hematology service with cytopenia. CD4 count was 1210 cell/ microL and HIV viral load was undetectable at <40 copies/mL. Patient had history of gut angiodysplasia with gastrointestinal bleeding previously treated with argon plasma coagulation endoscopically and octreotide. Complete blood count (CBC) showed white blood cell (WBC) count of 3.0 × 109/L, neutrophils between 0.3 × 109/L and 0.5 × 109/L, hemoglobin (Hb) of 6.5 g/L, mean corpuscular volume (MCV) of 101 fL and platelet count (PLT) of 80 × 109/L. Peripheral blood smear revealed presence of ovalomacrocytes, basophilic stippling and Howell-Jolly bodies in erythrocytes and neutrophils with nuclear hyposegmentation and hypogranulation (Pelger-Huet abnormalities). Nutritional deficiencies (iron, vitamin B12 and folic acid), renal or hepatic disorders as well as concomitant viral infections, were ruled out.
BM evaluation showed hypercellular marrow with trilineage dysplasia and 12% blasts some of them with Auer rods (Figure 1). Karyotype was normal. Molecular analysis for TP53 mutation was negative. The diagnosis was MDS with excess blast-2 (MDS-EB-2). International Prognosis Scoring System (IPSS) score was intermediate-2 risk and Revised-IPSS (R-IPSS) score was very high risk. He received transfusion support and started azacytidine treatment. Due to uncertainty as how it would be tolerated in the setting of HIV and ART the first 2 cycles of azacytidine were given at a dose of 75 mg/m2 for 5 days. Treatment was well tolerated and from third cycle on azacytidine was given at standard dose (75 mg/m2 for 7 days). He achieved partial response after 6 cycles of azacytidine (4% BM blast cells) with increased PLT. Neutropenia persisted without infectious complications. Anemia persisted with red blood cell transfusion dependence, but he had many gastrointestinal bleeding episodes due to angiodysplasia. After 13 azacytidine cycles he progressed to AML with a final CBC that showed WBC count of 7.0 × 109/L, neutrophils 0.1 × 109/L, Hb of 5.5 g/L and PLT count of 35 × 109/L. He received supportive care and died after a month. His progression free survival was 21 months with an overall survival of 24 months.
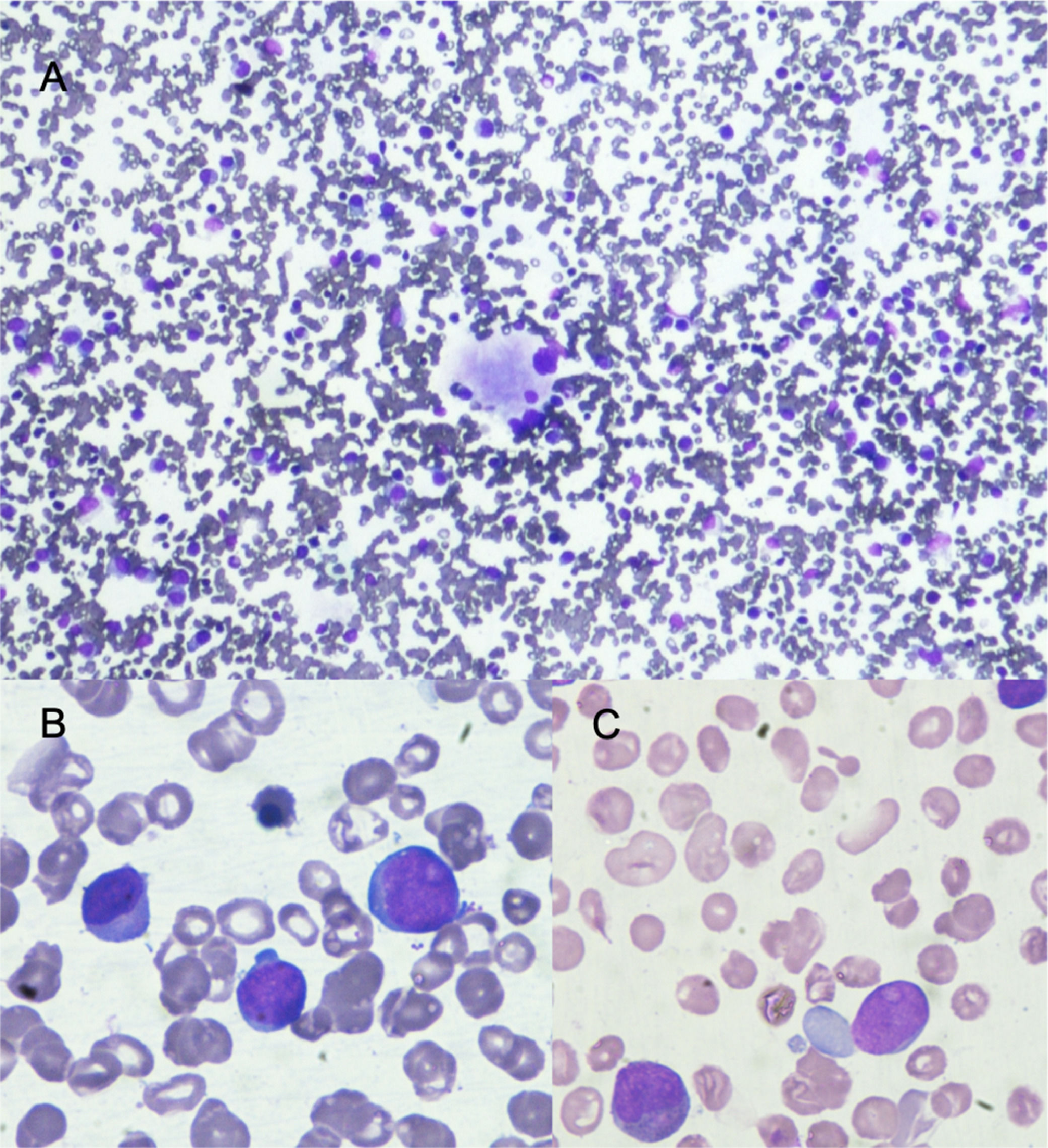
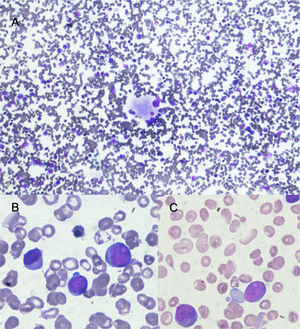
Bone marrow aspirate with May Grünwald Giemsa stain. A) 10x shows normocellular bone marrow with dysplastic megakaryocyte. B and C show in 100x large cells with high nuclear cytoplasmatic ratio, large nucleus with evident nucleoli these cells (blast cells) represent 12% of nucleated cells.
A 64-year-old Caucasian man, ex-alcoholic, heavy smoker. HIV infection diagnosis one year ago. Well-controlled on ART (lamivudine, tenofovir and dolutegravir) without AIDS-related illnesses. CD4 count of 693 cells/ microL and HIV viral load undetectable at <40 copies/mL. He was referred to our hematology service with 6 months transfusion dependent anemia. He presented with weakness, tiredness and dizziness. No history of bleeding, fever, infections or bone pain. CBC revealed Hb of 6.1 g/dL, MCV 119 fL, WBC 3.2 × 109/L, neutrophils 0.7 × 109/L and PLT of 170 × 109/L. Peripheral blood smear showed neutrophils with nuclear hyposegmentation and hypogranulation (Pelger-Huet abnormalities). No nutritional deficiencies. Normal liver and renal function. Hepatitis C virus, and Hepatitis B virus serological markers were negative. BM aspirate presented hypercellular marrow with trilineage dysplasia and 3% of myeloblasts without Auer rods (Figure 2). BM trephine sections were hypercellular with dysplastic change in megakaryocyte and granulocytic compartment. Myeloblast 5%. Karyotype: 46, XY,del(5)(q13q33)[2]/46,XY[9]. Fluorescent in situ hybridization (FISH) confirmed the presence of the 5q abnormality (33%, 66/200 analyzed nuclei). Erythropoietin (EPO) was below 500UI/L. The diagnosis was MDS with isolated del(5q). IPSS score was intermediate-1 risk and R-IPSS score was intermediate risk. He received first line treatment with EPO at 60.000UI/week and red blood cell transfusion support. After 8 weeks of treatment there was no response and transfusion dependency persisted. Lenalidomide was started according to recommendations on the use of lenalidomide in MDS (10 mg for 21 days every 28 days). He is on the 4th cycle and tolerating well. A complete hematologic response is achieved with red blood cell transfusion independence. Current CBC revealed Hb of 14.1 g/dL, MCV 100 fL, WBC 5.0 × 109/L, neutrophils 1.9 × 109/L and PLT of 105 × 109/L.
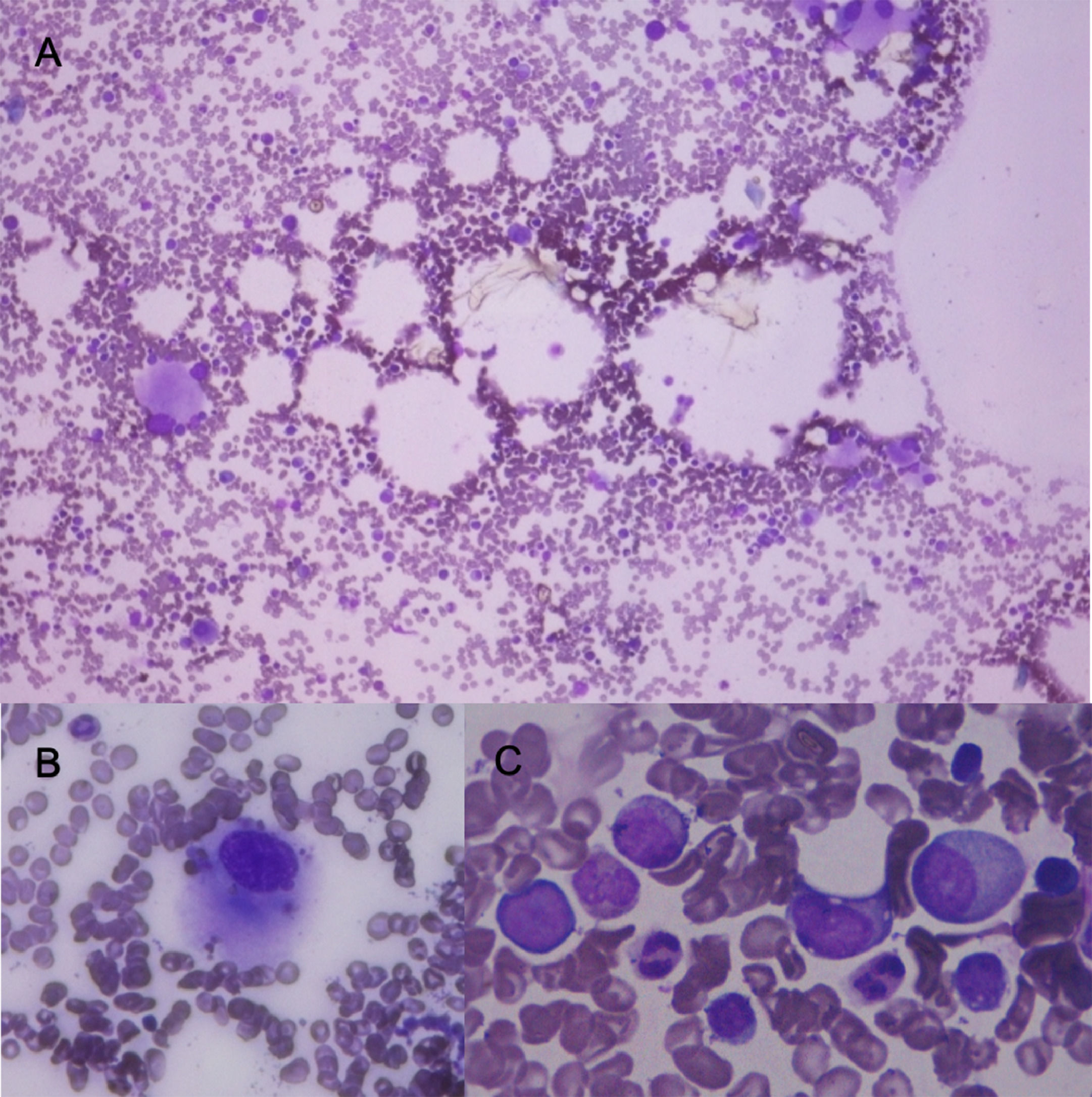
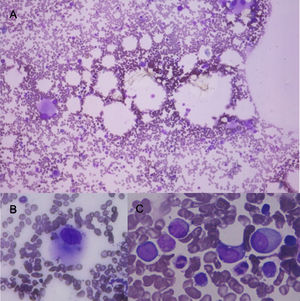
Bone marrow aspirate with May Grünwald Giemsa stain. A) 10x shows normocellular bone marrow with numerous dysplastic megakaryocytes. B) shows in 100x a dysplastic megakaryocyte. C shows in 100x granular dysplasia with hipogranulation, and large cells with high nuclear cytoplasmatic ratio, large nucleus and evident nucleoli (blast cells) these cells represent 3% of nucleated cells.
In 2019, an estimated 38.0 million people were living with HIV, two-thirds of whom were on ART. People living with HIV have higher risk of some cancers compared to general population.2 Since the introduction of ART in 1995, the incidence of acquired immunodeficiency syndrome (AIDS) defining cancers have dramatically decreased.2 However, longer life expectancy with ART and aging has led to increased incidence of non-AIDS defining cancers.2 MDS should be considered among non-AIDS-defining hematological malignancies, but the link between HIV and MDS is not fully elucidated yet. Jiamsakul et al. recently reported the incidence of malignancies in 7455 HIV Asian patients. One percent of patients (n=107) developed a malignancy, 31% of them were hematological and only one (0.9%) was MDS.4
Currently, MDS diagnosis according to World Health Organization criteria is based on clinical, cytological, histological, cytogenetical and molecular data.1 Prognosis is established by Revised International Prognostic Scoring System (R-IPSS) and other scores. Since cytopenia and marrow dysplasia frequent findings in HIV patients, MDS diagnosis is challenging, particularly in the absence of cytogenetic abnormalities, blast excess or ring sideroblasts. Patients we presented had blast excess and specific cytogenetic alteration respectively, making it easier to reach MDS diagnosis.
HIV associated cytopenia are more frequently observed in the context of uncontrolled viral replication and chronic infections. Neutropenia is the most common cytopenia, followed by anemia and thrombocytopenia. Etiology of cytopenia in this population is varied, including co-infections, nutritional deficiencies, drugs, comorbidities, hypersplenism, endocrinopathies, autoimmune diseases, as well as a direct effect of HIV in hematologic stem and progenitor cells (HSPC).5
BM dysplastic changes have been highly reported in association with HIV, making primary MDS diagnosis difficult. Frequent findings include varied degrees of dysplasia in one or more cell lines being erythroid dysplasia the most common, present in up to 50% of HIV patients. The etiology and implications BM dysplasia are not fully understood and there are no data that associate it with greater risk of developing MDS.5
Published data about MDS in HIV patients is scarce. Koichi et al. reported that MDS-HIV patients were relatively young and had complex and poor prognostic cytogenetic characteristics, including monosomy 7 and 7q deletion. Additionally, they had higher rate of AML transformation (63%) and poor overall survival compared to non-HIV patients.6 Moreover, Kaner et al. recently reported a small retrospective study showing that HIV-MDS patients presented at a younger age (59 vs. 71 years old, p<0.001) had higher risk disease, faster progression to AML, and worse overall survival (median survival 11.2 vs 69.1 mo, p<0.001) compared to non-HIV MDS patients. They also found that there was a higher prevalence of clonal-hematopoiesis related mutations (ASXL-1, DNMT3A) and a higher proportion of patients with high-risk cytogenetics. Both of the cases presented here are elderly patients with well-controlled HIV infection, one of them with a high-risk disease that progressed to AML and the other with intermediate risk MDS that is currently receiving treatment. The fact that viral infection was well controlled in both patients raises the question whether HIV infection played an important role in MDS development or if these should be considered de novo diseases. However, when viral replication is controlled chronic inflammation still persists playing an important role in immune deregulation that leads to myelodysplasia. Although next generation sequencing (NGS) was not performed at the time of diagnosis, due to a lack of availability in our institution. Our laboratory is setting this technology in the near future and as we have both patient's DNA saved we will be able to study them.
Hypomethylating agents 5-azacytidine and decitabine are the current standard of care for patients with high-risk MDS based on the results of the MDS AZA-001 trial.7 With regards to hypomethylating treatment in the setting of HIV infection and ART evidence is limited and mainly based on case reports.3 One of our patients was treated with azacytidine with a reduced dose in the first two cycles and since he tolerated well, full dose was indicated in the following cycles. Partial remission was achieved and maintained until he progressed to AML after 13th cycle and died. This case demonstrates safe use of azacytidine for MDS in an HIV-infected patient on ART.
Lenalidomide is the treatment of choice for low risk MDS with 5q deletion after erythropoiesis stimulating agent failure in transfusion dependent patients achieving RBC-transfusion independence in near 75% with median response duration of 2–3 years. Lenalidomide has been successfully used in HIV patients for hematological and non-hematological malignancies.8,9 Most common adverse event was myelosupression, comparable to reports in non-HIV population. There is limited data regarding the use of Lenalidomide in HIV-MDS. Blum et al. reported the case of an HIV+ patient with 5q- MDS syndrome who received Lenalidomide together with ART, with good tolerance.9 Additionally, Kaner et al. reported 2 cases of HIV-MDS treated with Lenalidomide. However, response and toxicity profile were not specified.3 In our case, patient started Lenalidomide while on ART and he is currently receiving his third cycle with good tolerance and without myelosuppression.
The pathophysiologic mechanisms of HIV-associated MDS are not completely elucidated to the date. The development of clonal hematopoiesis of indeterminate potential (CHIP) is in origin of MDS. Increasing evidence links inflammation and clonal development of CHIP-related mutations. Moreover, niche plays an important role in allowing the outgrowth of mutated clones over normal hematopoiesis. There is vast evidence demonstrating that HIV infection in the setting of ART is commonly associated with chronic inflammation. Bick et al. have recently reported that HIV is associated with at least 2 fold increased prevalence of CHIP10 and this could be the basis of MDS development. One possible hypothesis for the increased CHIP prevalence in HIV+ patients and MDS development is that viral infection modified BM niche by an inflammatory milieu predisposing to somatic mutation acquisition. Additionally, comorbidities, concomitant viral infections, and ART may have a role in somatic mutations acquisition in HSPC.
In conclusion, data linking HIV infection and MDS is scarce. Moreover, numbers of HIV-infected patients with MDS are insufficient to allow high-quality studies. Therefore, case reports and series may represent the best available evidence to aid us in our clinical practice. We presented two cases at our MDS clinic. That, represents a prevalence of 2.7% in our registry (n=75). Presented cases highlight the importance of MDS diagnosis in HIV setting. Besides they suggest the use of azacytidine and lenalidomide is safe in HIV infected patients on ART.