Venetoclax-based regimens have emerged as a standard therapeutic option for newly diagnosed Acute Myeloid Leukemia (AML) in patients deemed unfit for intensive chemotherapy. However, the efficacy of venetoclax in fit patients remains an area of ongoing investigation. Notably, in specific AML subsets, such as Core Binding Factor (CBF) AML, venetoclax-based therapy has demonstrated inferior outcomes compared to intensive chemotherapy. Despite these findings, direct comparative data between venetoclax-based therapies and intensive induction chemotherapy in fit patients with non-CBF AML remains limited. This study aims to evaluate and compare the clinical outcomes of fit younger adult patients with newly diagnosed non-CBF AML who underwent induction therapy with either venetoclax-based regimens or standard 7+3 chemotherapy.
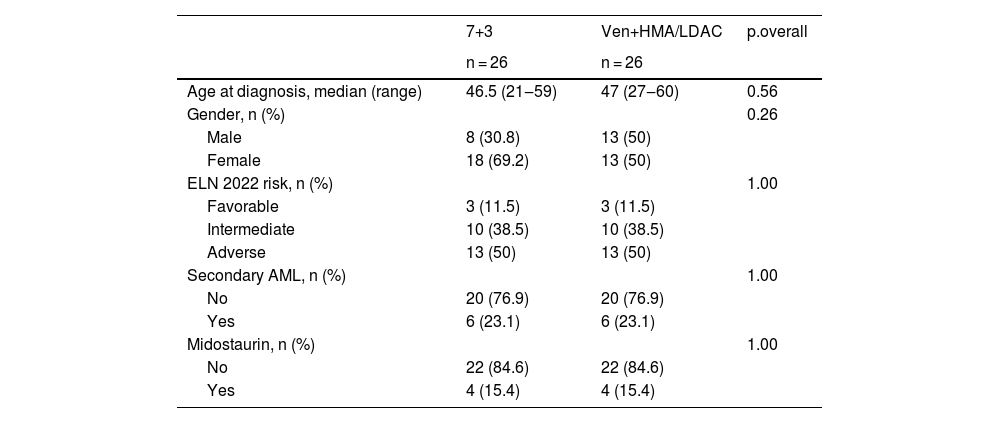
MethodologyThis retrospective cohort study included patients assessed at RM Gorbacheva Research Institute for eligibility for Allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT) from June 2020 to August 2024. Eligible patients were adults with non-CBF AML who received either 7+3 induction chemotherapy or venetoclax in combination with a Hypomethylating Agent (HMA) or Low-Dose Cytarabine (LDAC). Exclusion criteria included age > 60-years and a Hematopoietic Cell Transplantation-Specific Comorbidity Index (HCT-CI) score > 2. To minimize confounding, pairwise propensity score matching was performed based on age, secondary AML status, and ELN 2022 risk classification. Remission in this study referred to Complete Remission (CR), CR with partial or incomplete hematologic recovery, and a morphological leukemia-free state according to the ELN response criteria. Patients who failed to achieve remission after two induction cycles were categorized as refractory. Overall Survival (OS) was defined as the time from start of treatment to death from any cause. Event-Free Survival (EFS) included refractoriness, relapse, or death, with censoring at the last follow-up. Relapse was defined as the reappearance of ≥5% blasts in bone marrow or peripheral blood, or extramedullary disease. Non-Relapse Mortality (NRM) was defined as death in remission. Survival analysis was conducted using the Kaplan-Meier method and log-rank test. Cumulative incidences of relapse and NRM were assessed using competing risk models with Gray’s test. Statistical analyses were performed using R (version 4.4.2). The study adhered to the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the Pavlov University Ethical Committee.
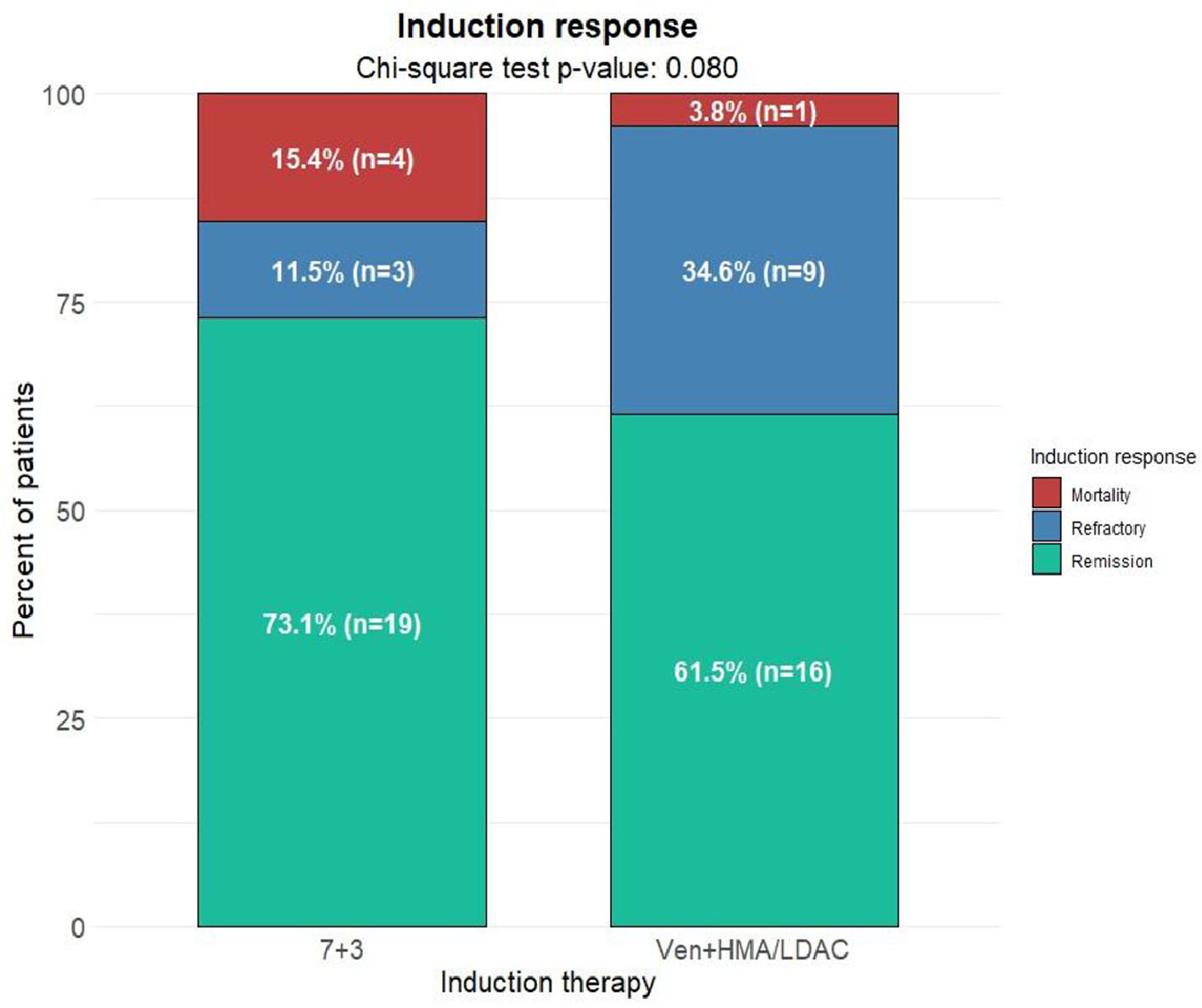
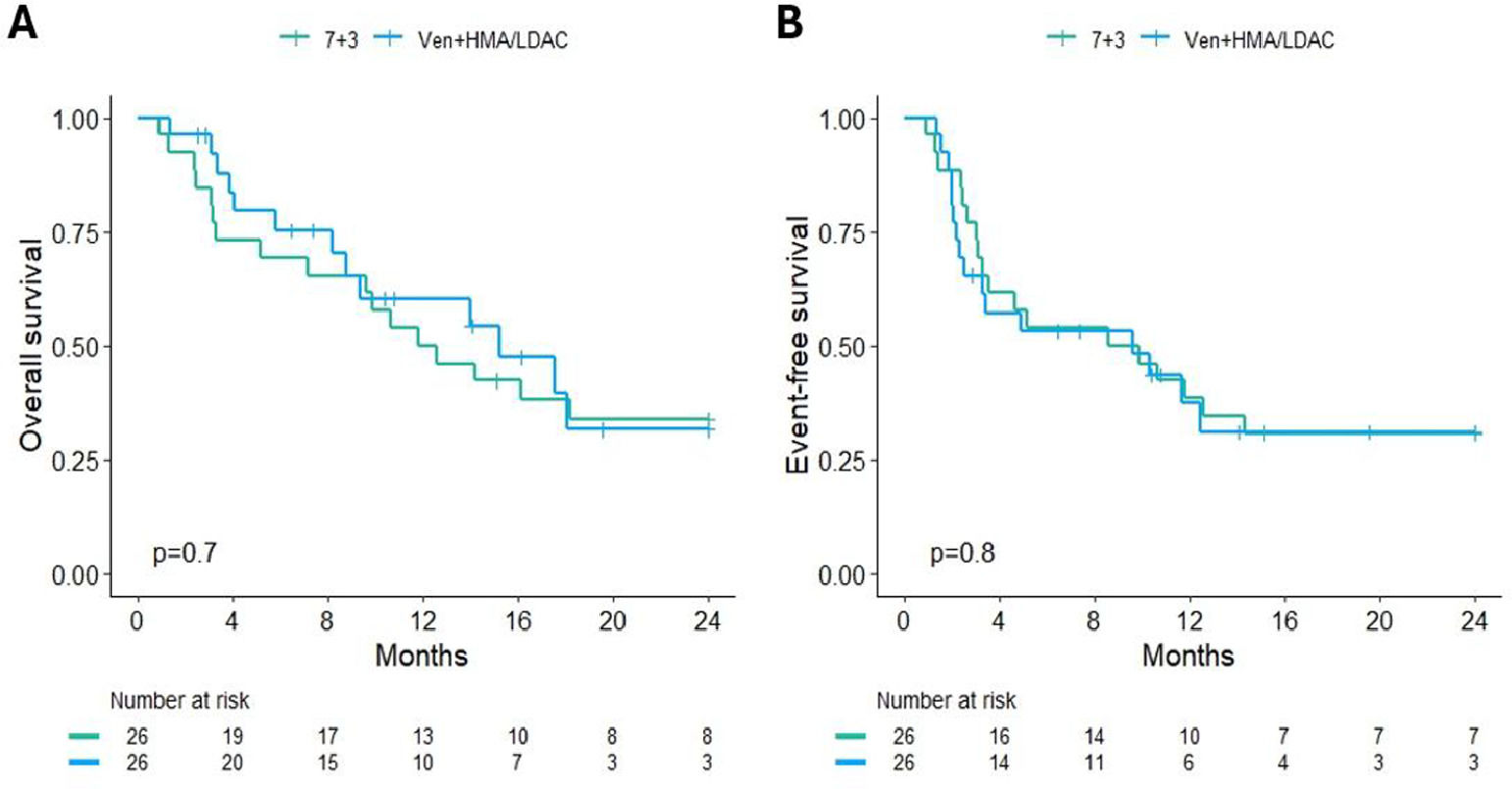
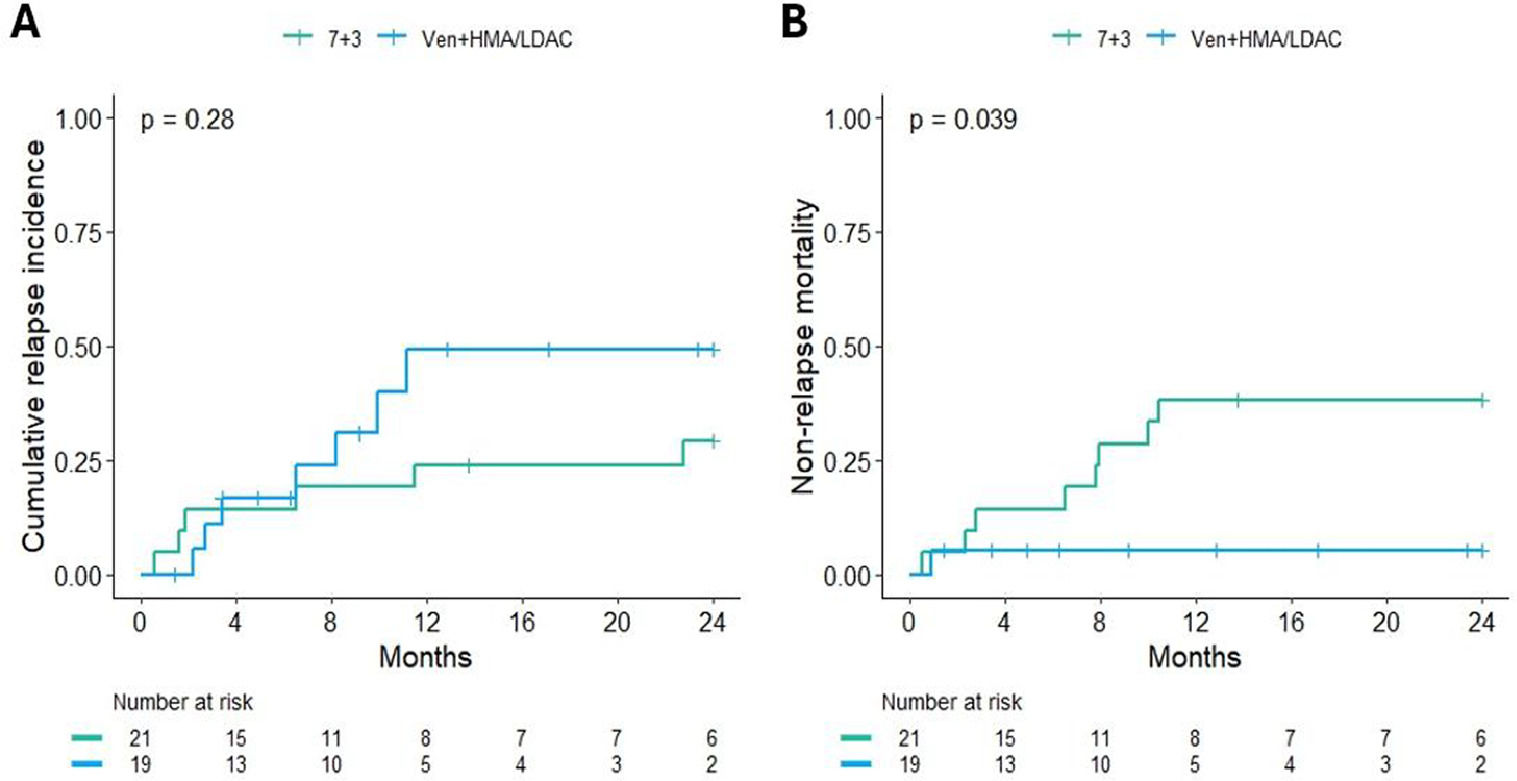
ResultsA total of 112 patients met the inclusion criteria, with 64.3% (n = 72) receiving 7+3 induction and 35.7% (n = 40) treated with venetoclax plus HMA/LDAC. After propensity score matching, each treatment arm included 26 patients. Baseline characteristics of the matched cohort are summarized in Table 1. Remission rates were 73.1% (n = 19) in the 7+3 group and 61.5% (n = 16) in the venetoclax group. Refractory disease was documented in 11.5% (n = 3) and 34.6% (n = 9), respectively. Induction-related mortality occurred in 15.4% (n = 4) of the 7+3 group and 3.8% (n = 1) of the venetoclax group (p = 0.08) (Fig. 1). The median follow-up for surviving patients was 25.5 months (range: 2.5‒37.9). Two-year OS rates were 33.8% (95% CI: 19.6‒58.4) for the 7+3 group and 31.6% (95% CI: 15.1‒66.2) for the venetoclax group (p = 0.7). Two-year EFS was 30.8% (95% CI: 17.3‒54.8) and 31% (95% CI: 16.1‒59.8), respectively (p = 0.8) (Fig. 2). Cumulative relapse incidence was 29% (95% CI: 11‒50) in the 7+3 group and 49% (95% CI: 19‒74) in the venetoclax group (p = 0.28). NRM was significantly higher in the 7+3 group at 38% (95% CI: 18‒58) compared to 5.3% (95% CI: 0.3‒22) in the venetoclax group (p = 0.039) (Fig. 3). The cumulative incidence of allo-HSCT was 46% (95% CI: 26‒64) and 55% (95% CI: 30‒75) for 7+3 and venetoclax groups, respectively (p = 0.15).
ConclusionIn this propensity-matched analysis of fit younger adults with non-CBF AML, venetoclax-based induction therapy demonstrated comparable overall and event-free survival to standard 7+3 chemotherapy. While venetoclax-treated patients exhibited a numerically higher relapse incidence, this difference did not reach statistical significance. Conversely, those receiving 7+3 experienced significantly greater non-relapse mortality. Notably, venetoclax-based therapy was associated with a lower induction-related mortality and a higher rate of refractory disease, underscoring the distinct response dynamics of these regimens. These findings highlight the nuanced risk-benefit profiles of venetoclax and intensive chemotherapy, warranting further prospective validation to optimize patient selection and treatment strategies in fit AML patients.
Table 1 Baseline characteristics of the matched cohort.