Hematopoietic stem cell transplantation has been successfully used to treat the pediatric population with malignant and non-malignant hematological diseases. This paper reports the results up to 180 days after the procedure of all unrelated hematopoietic stem cell transplantations in pediatric patients that were performed in one institution.
MethodsA retrospective review was performed of all under 18-year-old patients who received unrelated transplantations between 1995 and 2009. Data were analyzed using the log-rank test, Cox stepwise model, Kaplan–Meier method, Fine and Gray model and Fisher's exact test.
ResultsThis study included 118 patients (46.8%) who received bone marrow and 134 (53.2%) who received umbilical cord blood transplants. Engraftment occurred in 89.47% of the patients that received bone marrow and 65.83% of those that received umbilical cord blood (p-value<0.001). Both neutrophil and platelet engraftments were faster in the bone marrow group. Acute graft-versus-host disease occurred in 48.6% of the patients without statistically significant differences between the two groups (p-value=0.653). Chronic graft-versus-host disease occurred in 9.2% of the patients with a higher incidence in the bone marrow group (p-value=0.007). Relapse occurred in 24% of the 96 patients with malignant disease with 2-year cumulative incidences of 45% in the bone marrow group and 25% in the umbilical cord blood group (p-value=0.117). Five-year overall survival was 47%, with an average survival time of 1207 days, and no significant differences between the groups (p-value=0.4666).
ConclusionDespite delayed engraftment in the umbilical cord blood group, graft-versus-host disease, relapse and survival were similar in both groups.
Allogeneic hematopoietic stem cell transplantation (HSCT) is an accepted treatment for a number of inherited and acquired hematopoietic diseases in children, especially diseases for which an alternative treatment is not available or no longer effective.1,2
Human leukocyte antigen (HLA)-matched sibling donors are available only for around 25% of such children. However, there has been substantial progress over the last four decades in the use of alternative donors, including unrelated volunteer donors.3–5
The known advantages of unrelated umbilical cord blood (UCB) over unrelated bone marrow (BM) are well documented and include: significantly faster availability of banked cryopreserved UCB units, no risk to the donor, reduced transmission of viral illnesses such as cytomegalovirus (CMV) and Epstein Barr virus (EBV), tolerance of HLA disparity between the donor and recipient, and reduced risk and severity of acute graft-versus-host disease (GVHD). However, the main problem of using UCB for transplantation is the low number of hematopoietic progenitor cells, which results in increased risk of graft failure, delayed hematopoietic engraftment and delayed immune reconstitution.6,7
HLA matching, the use of high-dose chemotherapy and/or radiotherapy and the need of immunosuppressive drugs represent the causes of onset of many complications following HSCT.8,9
GVHD is a common complication, mainly in unrelated HSCT or in the presence of any HLA allele mismatch.10 At an incidence of 40%, acute GVHD occurs in the early period after transplant with the skin being the most commonly affected tissue. Risk factors for the development of GVHD include the recipient's age, CMV serostatus, HSC donor source and HLA disparity.11,12 Chronic GVHD is observed in 30–90% of recipients of HSCT.3
In patients with malignant diseases, GVHD is associated with the graft-versus-leukemia effect (GVL), thus resulting in a decreased incidence of relapse.13,14
In recent decades, the overall survival (OS) in children who received HSCT is much higher, about 60% one to two years after transplant, depending on the disease, clinical conditions prior to transplant and complications after the transplant.2,6 Therefore, this study aimed to analyze the results of unrelated HSCT in pediatric patients up to 180 days after the procedure and to compare the stem cell sources used.
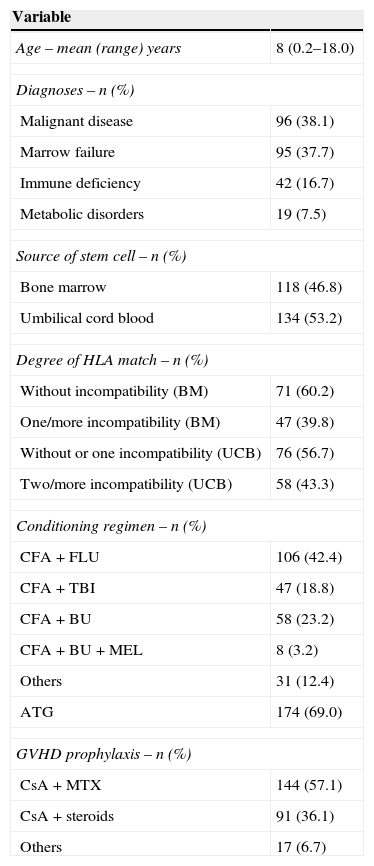
MethodsPatientsThis is a retrospective study. Between January 1995 and December 2009, 261 under 18-year-old patients received unrelated HSCT at the Hospital de Clínicas, Universidade Federal do Paraná (UFPR), Brazil. Of the 261 patients, nine were excluded from the analysis for the following reasons: seven patients had insufficient data for analysis and two patients were recipients of peripheral blood transplants. One hundred and eighteen patients received BM and 134 UCB grafts. The characteristics of the 252 cases that were assessed are listed in Table 1.
Features of patients and treatment.
| Variable | |
|---|---|
| Age – mean (range) years | 8 (0.2–18.0) |
| Diagnoses – n (%) | |
| Malignant disease | 96 (38.1) |
| Marrow failure | 95 (37.7) |
| Immune deficiency | 42 (16.7) |
| Metabolic disorders | 19 (7.5) |
| Source of stem cell – n (%) | |
| Bone marrow | 118 (46.8) |
| Umbilical cord blood | 134 (53.2) |
| Degree of HLA match – n (%) | |
| Without incompatibility (BM) | 71 (60.2) |
| One/more incompatibility (BM) | 47 (39.8) |
| Without or one incompatibility (UCB) | 76 (56.7) |
| Two/more incompatibility (UCB) | 58 (43.3) |
| Conditioning regimen – n (%) | |
| CFA+FLU | 106 (42.4) |
| CFA+TBI | 47 (18.8) |
| CFA+BU | 58 (23.2) |
| CFA+BU+MEL | 8 (3.2) |
| Others | 31 (12.4) |
| ATG | 174 (69.0) |
| GVHD prophylaxis – n (%) | |
| CsA+MTX | 144 (57.1) |
| CsA+steroids | 91 (36.1) |
| Others | 17 (6.7) |
CFA: cyclophosphamide; FLU: fludarabine; TBI: total body irradiation; BU: busulfan; MEL: melphalan; ATG: anti-thymocyte globulin; CsA: cyclosporine; MTX: methotrexate.
The units were selected on the basis of best HLA matching and a critical minimum cell dose at the discretion of the treating physicians and source from National and International Public Banks. Class I typing was performed by serological or molecular techniques and Class II typing by molecular techniques; it was only in 2008 that the analysis of the locus C was frequently performed.
Conditioning regimen and prophylaxis against graft-versus-host diseaseThe conditioning regimen and prophylaxis for acute GVHD varied according to the underlying disease, stem cell source and HLA incompatibilities (Table 1).
TransplantationThe units of BM grafts used for transplantation contained an average of 4.39×108 total nucleated cells (TNC) (range: 0.3–10.8 cells) per kilogram of recipient's body weight after thawing. The units of UCB grafts used for transplantation contained an average of 5.2×107 TNC (range: 1.4–36.5 cells) and 1.3×105 CD34 cells (range: 0.1–11.4 cells) per kilogram of recipient's body weight after thawing.
Supportive careCentral venous catheters were inserted in all patients. Patients received acyclovir for antiviral prophylaxis, fluconazole or amphotericin-B for antifungal prophylaxis, and trimethoprim-sulfamethoxazole for prophylaxis against Pneumocystis carinii. Empirical broad spectrum antibiotic therapy was started at the first sign of fever.
Hematopoietic recovery and engraftmentHematologic recovery was defined as the time when the absolute neutrophil count was equal to or greater than 0.5×109cells/L in three consecutive laboratory counts on different days and platelet count equal to or greater than 20×109cells/L (after seven days without transfusion support).6
Graft-versus-host diseasePatients were evaluated and considered at risk for acute GVHD when there was evidence of neutrophil recovery. Diagnosis of acute GVHD was based on clinical criteria, with histopathologic confirmation when possible. Overall staging was assessed according to previously published criteria.15 Only those patients with sustained engraftment of donor hematopoiesis surviving for more than 90 days after transplant were assessed for chronic GVHD according to the criteria described in previous studies.16
Other measurementsRelapse was characterized by morphological evidence of malignant disease at any site. Transplant-related mortality (TRM) was determined to be every cause of death that was not death by relapse. OS was the time between transplantation and death due to any cause or between transplantation and the day of the last follow-up.
Statistical analysisAll statistical analyses were carried out using the STATA v.12.0 computer program. Survival rates were calculated using the Kaplan–Meier method. The log-rank test was used for testing differences in survival between groups in the univariate analysis. Cumulative incidence curves were used for engraftment and GVHD endpoints with death as a competing risk factor. The Cox proportional hazards model with backward elimination was used for multivariate testing of co-variables (statistical significance was based on p-values≤0.05). Differences between subgroups were compared using the Fine and Gray test with death as a competing risk. The association between dichotomous variables was calculated using Fisher's exact test.
ResultsPatient characteristicsFrom 1995 to 2009, 252 patients were treated with unrelated HSCT. The mean age of the patients was eight years (range: 0.2–18 years) with 34 patients being one year old or less. Most patients were male (61.1%). A total of 96 patients were transplanted for malignant diseases with 64 (66.7%) being at an early stage. A total of 150 (59.5%) donor-graft pairs were matched for ABO and 124 (49.8%) were matched for gender. BM was used for HSCT in 118 patients (46.8%) and UCB in 134 patients (53.2%). The patients as well as treatment are listed in Table 1.
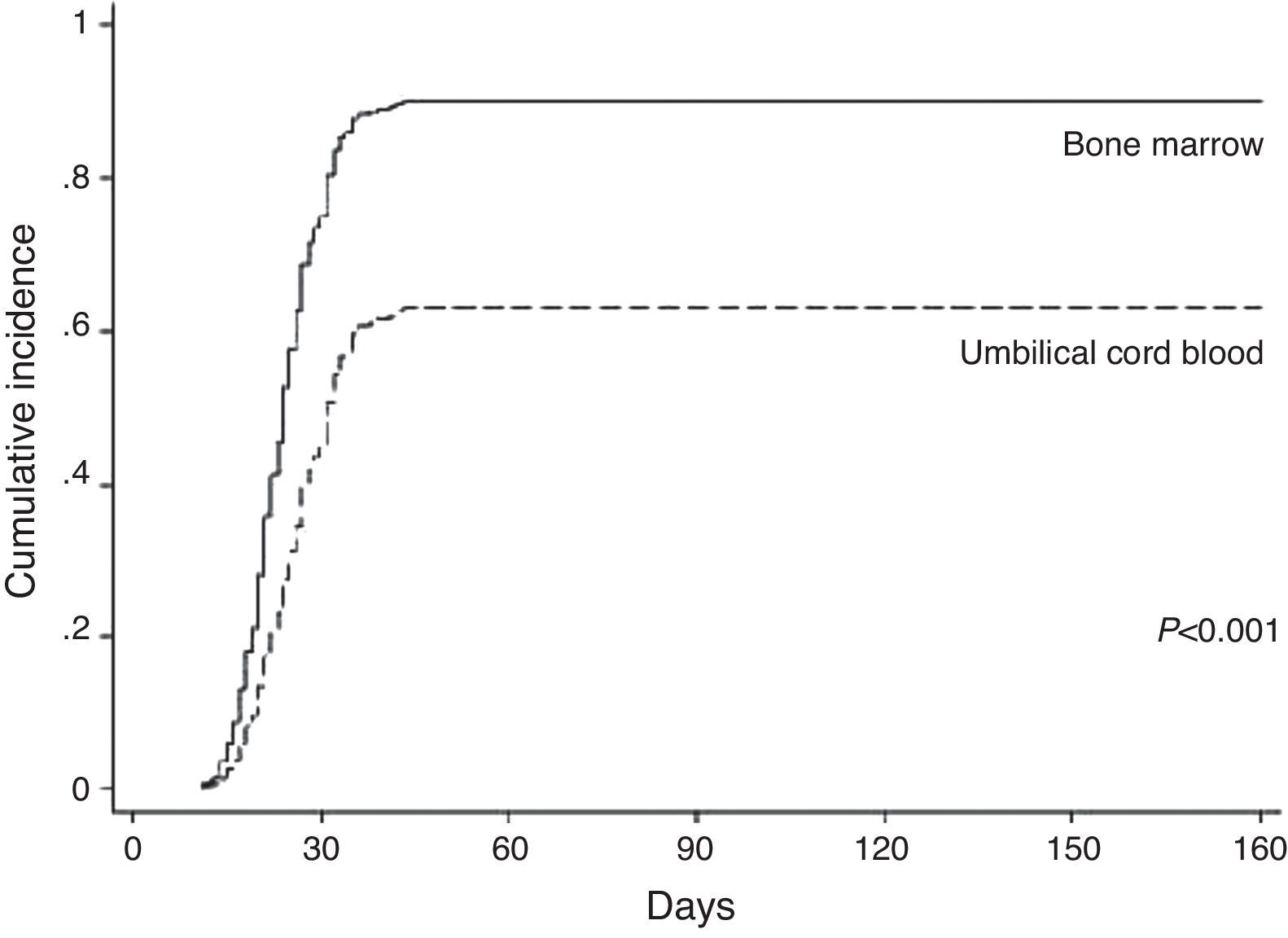
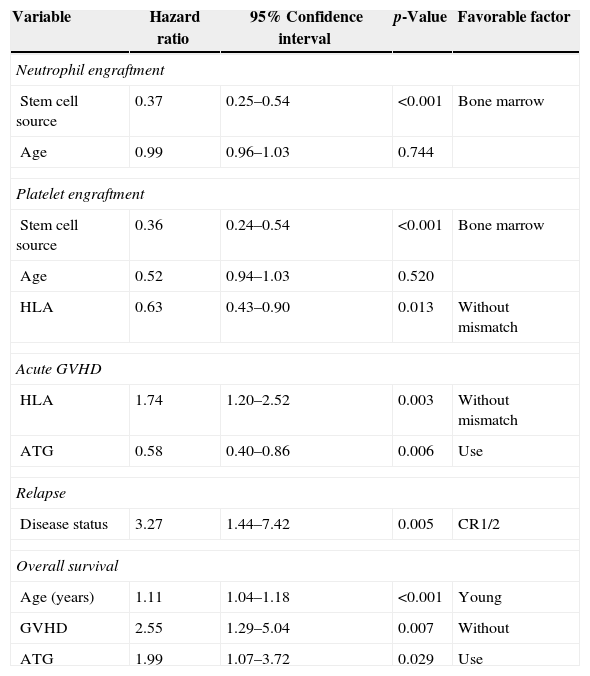
Neutrophil and platelet engraftmentNeutrophil engraftment occurred in 181 of the 234 (77.4%) avaliable (18 patients died before) cases (BM: 89.47% and UCB: 65.83%). In the BM group, 102 of the 118 patients achieved neutrophil engraftment in an average time of 24 days (range: 11–1696 days). The cumulative incidence by Day 30 was 80%. In the UCB group, 79 of the 134 patients achieved neutrophil engraftment in a mean time of 32 days (range: 14–4037 days). The cumulative incidence by Day 42 was 62%. In the univariate analysis, neutrophil engraftment was influenced by cell source (p-value<0.001) and patient age (p-value=0.002). In the multivariate analysis only cell source (p-value<0.001) was a significantly favorable factor for neutrophil engraftment (Table 2 and Figure 1).
Multivariate analysis.
| Variable | Hazard ratio | 95% Confidence interval | p-Value | Favorable factor |
|---|---|---|---|---|
| Neutrophil engraftment | ||||
| Stem cell source | 0.37 | 0.25–0.54 | <0.001 | Bone marrow |
| Age | 0.99 | 0.96–1.03 | 0.744 | |
| Platelet engraftment | ||||
| Stem cell source | 0.36 | 0.24–0.54 | <0.001 | Bone marrow |
| Age | 0.52 | 0.94–1.03 | 0.520 | |
| HLA | 0.63 | 0.43–0.90 | 0.013 | Without mismatch |
| Acute GVHD | ||||
| HLA | 1.74 | 1.20–2.52 | 0.003 | Without mismatch |
| ATG | 0.58 | 0.40–0.86 | 0.006 | Use |
| Relapse | ||||
| Disease status | 3.27 | 1.44–7.42 | 0.005 | CR1/2 |
| Overall survival | ||||
| Age (years) | 1.11 | 1.04–1.18 | <0.001 | Young |
| GVHD | 2.55 | 1.29–5.04 | 0.007 | Without |
| ATG | 1.99 | 1.07–3.72 | 0.029 | Use |
HLA: human leukocyte antigen; GVHD: graft-versus-host disease; ATG: anti-thymocyte globulin; CR1/2: complete remission 1/2.
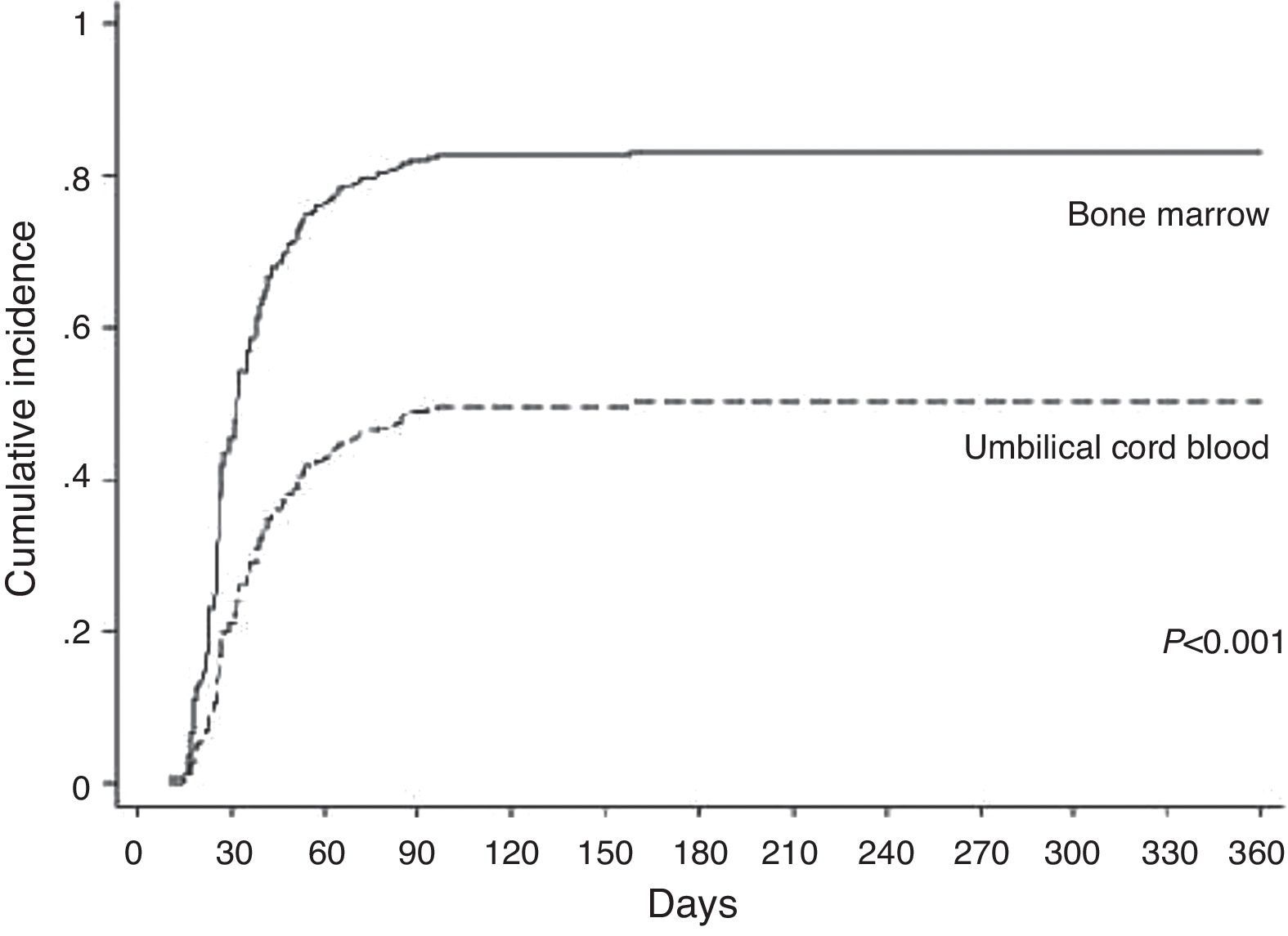
In the BM group, patients achieved platelet engraftment in an average of 25 days (range: 10–96 days). The cumulative incidence by Day 30 was 50%. In the UCB group, patients achieved platelet engraftment in a mean time of 43 days (range: 19–158 days). The cumulative incidence by Day 42 was 38%. In the univariate analysis, platelet engraftment was influenced by cell source (p-value<0.001), patient age (p-value=0.022) and HLA match (p-value=0.027). In the multivariate analysis cell source (p-value<0.001) and HLA match (p-value=0.013) were significantly favorable factors for platelet engraftment (Table 2 and Figure 2).
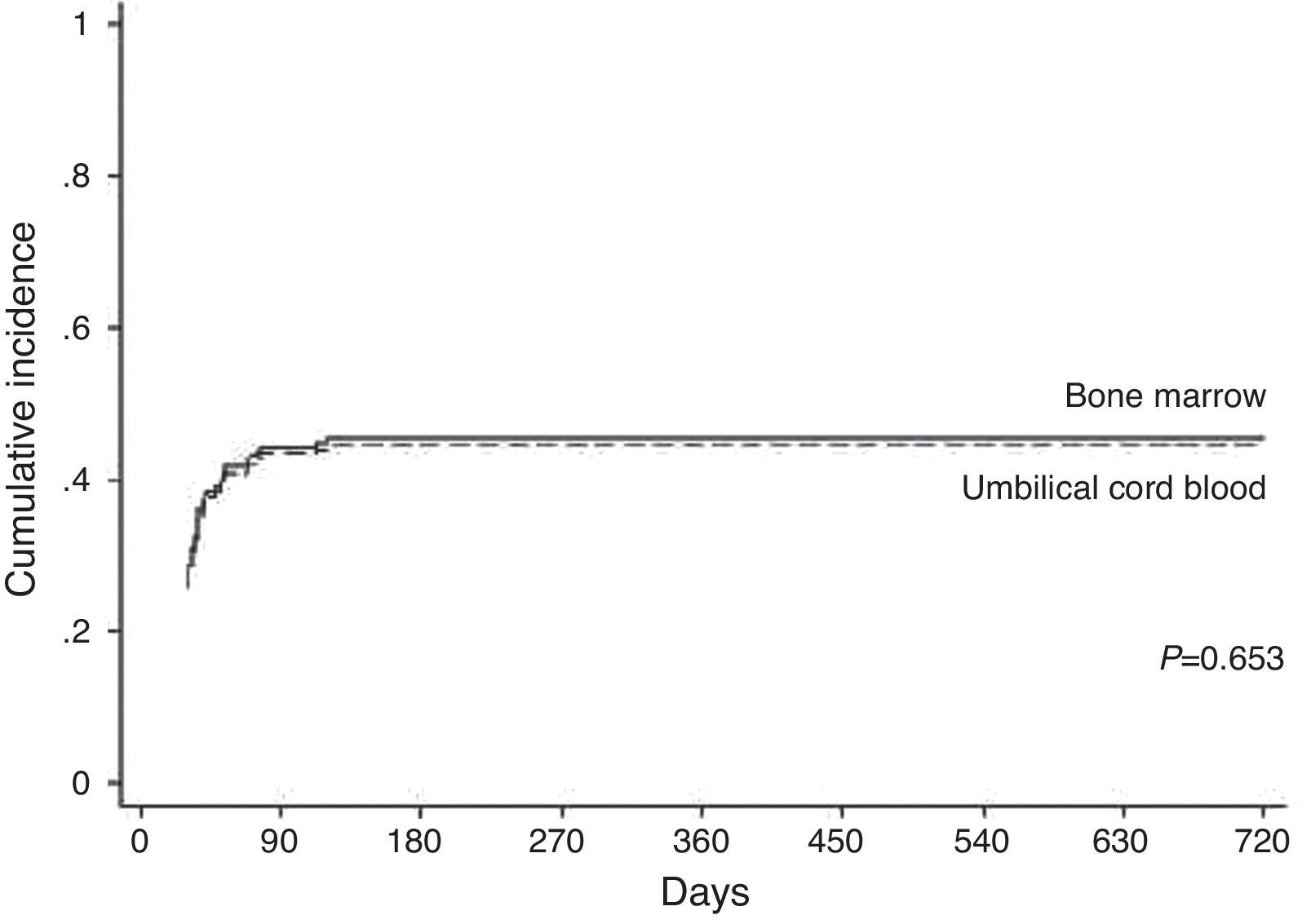
Graft-versus-host diseaseAcute GVHD occurred in 88 (48.6%) patients of the total of 181 (on average within 90 days) and was scored as Grade II (54.5%) and Grade III–IV (45.5%). By Day 100 after transplantation, the cumulative incidence was 44% in the BM group and 43% in the UCB group (p-value=0.653 – Figure 3). HLA match (p-value=0.004) and ATG (p-value=0.005) were significant in the univariate analysis; this was the same in the multivariate analysis (p-value=0.003 and p-value=0.006, respectively – Table 2).
Chronic GVHD occurred in 13 (9.2%) patients out of the total of 142 (on average within 90 days). The disease was classified as limited in six cases (46.2%) and extensive in seven cases (53.8%). At one year after transplantation, the cumulative incidence was 14% in the BM group and 2.5% in the UCB group (p-value=0.053).
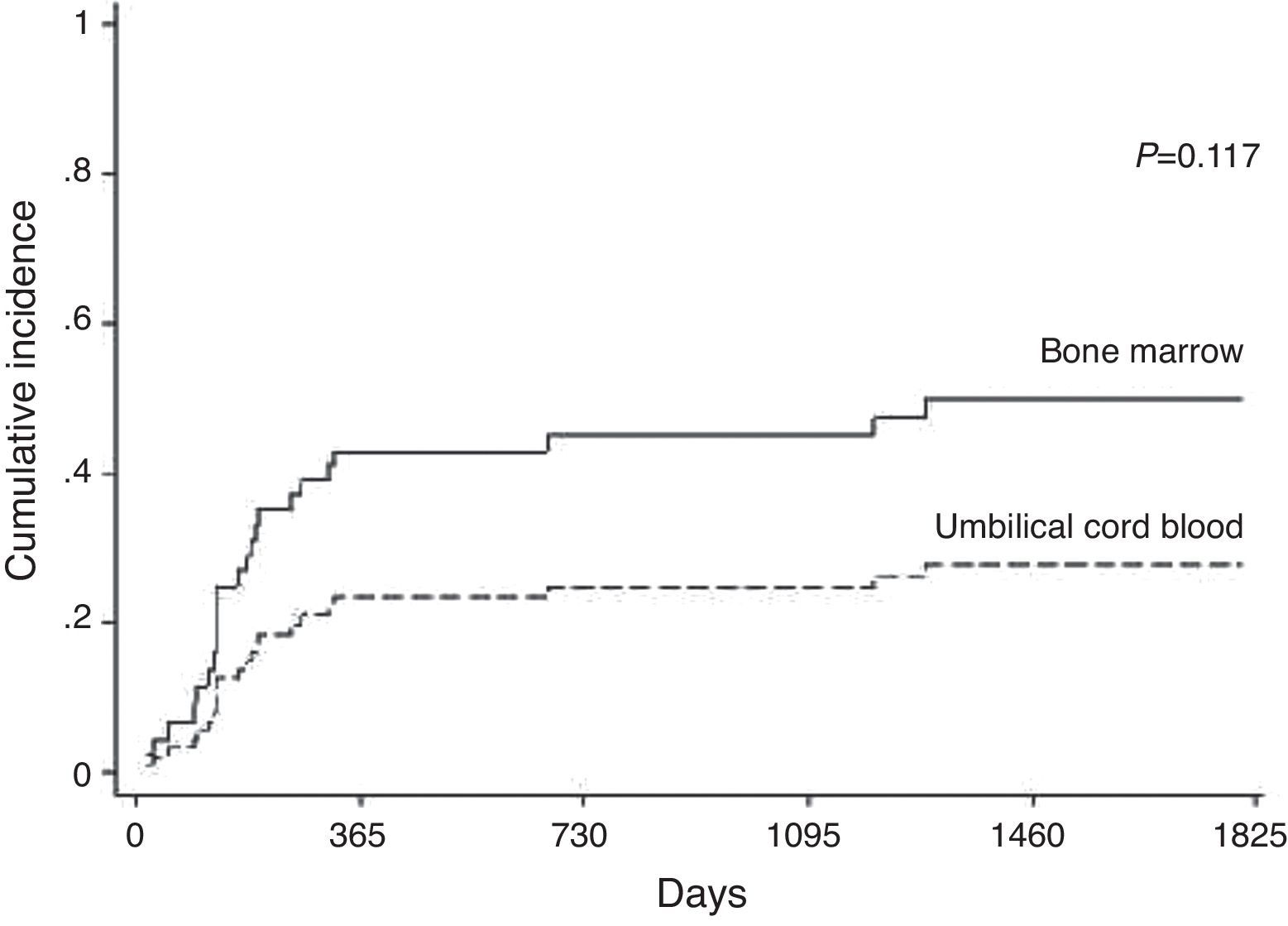
RelapseHematological relapse was detected between 21 and 1228 days (mean: 181 days; 95% confidence interval: 94.0–670.0) after transplantation in 23 of 96 patients treated for malignant disease. Relapse was observed in 29.31% of the patients in the BM group and 15.79% in the UCB group (p-value=0.117). The cumulative incidences of relapse were 45% in the BM group and 25% in the UCB group at two years (Figure 4). The stage of the disease was found to be significant (p-value=0.005) however cell source, GVHD, CMV and ABO incompatibility were not (Table 2).
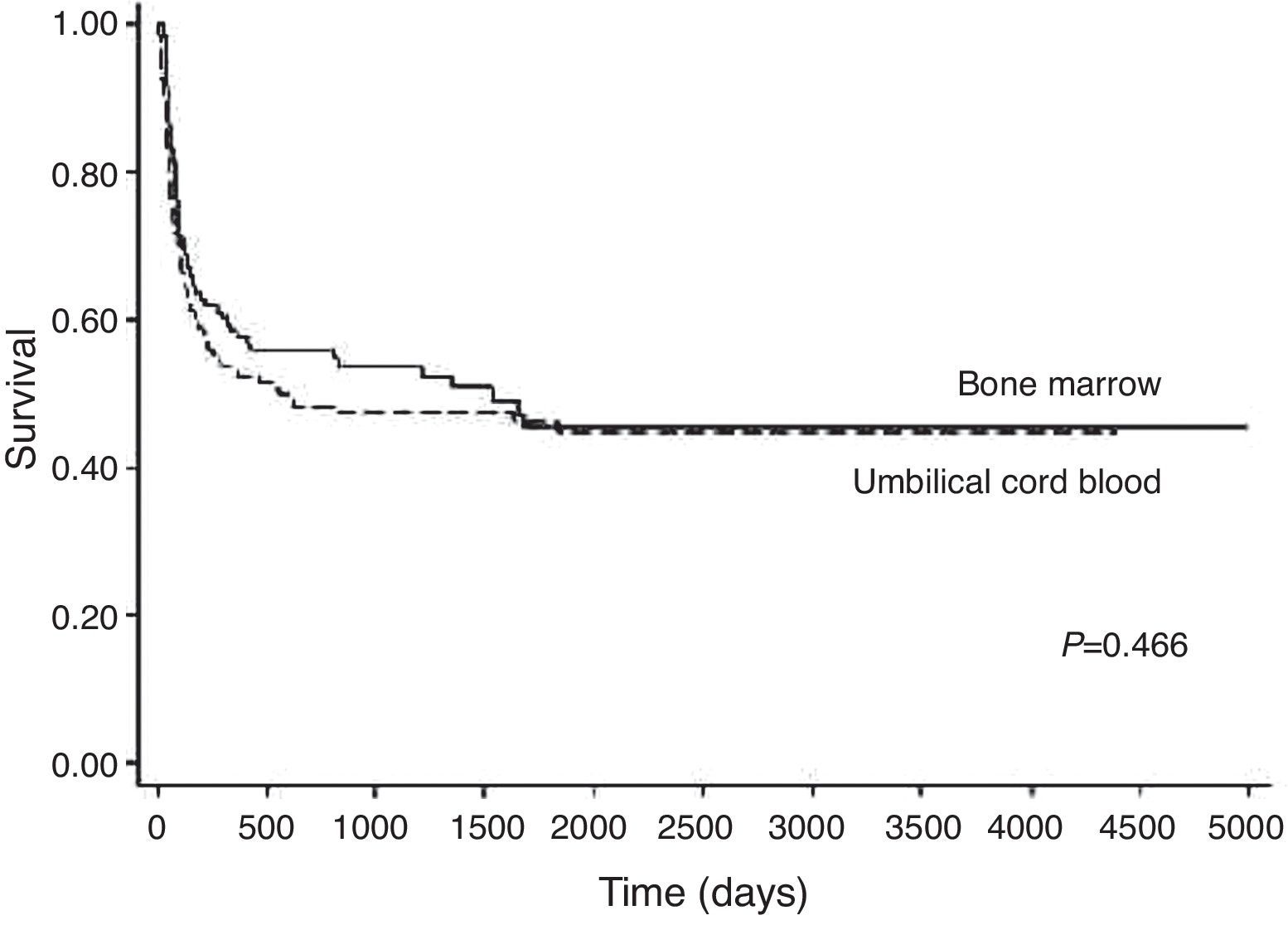
Overall survival and causes of deathWith an average follow-up time of surviving patients of 1207 days (3.3 years), the cumulative proportions of surviving patients at one year and five years were 55% and 47%, respectively. The cumulative proportions of surviving patients at one year and five years after unrelated HSCT with BM were 55% and 45%, respectively and for UCB they were 52% and 45%, respectively (p-value=0.466) (Figure 5). In a multivariate analysis, the factors that most influenced of negative form the OS were higher ages (p-value<0.001), non-use of ATG (p-value=0.029), and GVHD (p-value=0.007 – Table 2).
In this series, 131 (52.0%) patients died, 59 (50.0%) in the BM group and 72 (53.73%) in the UCB group (p-value=0.614). Death was associated with infection (n=47), graft failure (n=29), GVHD (n=25), relapse (n=18) and others (n=12).
DiscussionThis retrospective study compared a heterogeneous pediatric population with different diagnoses transplanted over a long period of time using two different stem cell sources. This study aimed to evaluate the outcomes of unrelated HSCT in the pediatric population comparing the use of BM and UCB; only 25% of patients who need transplants find suitable family donors.
The cumulative incidence (80% by Day 30 in the BM group, and 62% by Day 42 in the UCB group) and speed of neutrophil engraftment (on average by Day 24 in the BM group and Day 32 in the UCB group) were similar to the results of Barker et al.17 In other studies on UCB transplantations6,18,19 neutrophil engraftment was found higher and faster than in the current study. In this study, neutrophil engraftment was not influenced by HLA matching similar to the study of Petterson et al.6 even though Kurtzberg et al.18 found that HLA matching influenced neutrophil engraftment. It is known that, during the time of this study, HLA typing techniques were improved. Many studies1,6,19 demonstrated that the dose of infused CD34 cells influences neutrophil engraftment, however no difference was seen in the current study perhaps due to the low mean cell count infused.
The cumulative incidence (50% by Day 30 in the BM group, and 38% by Day 42 in the UCB group) and speed (on average, Day 25 in the BM group and Day 43 in the UCB group) of platelet engraftment were similar to Barker et al.12 Petterson et al.,6 who analyzed patients who received UCB transplantation, found that platelet engraftment was similar to this study. The authors reported that engraftment was not influenced by the infused TNC or CD34 cell dose or HLA matching. However in the current study, platelet engraftment was influenced by HLA matching. It is again important to note that HLA typing techniques were improved during this period.
Acute GVHD remains a major cause of morbidity and mortality after HSCT. The incidence of Grade II–IV GVHD is between 20 and 50%. The recipient's age, CMV serostatus, donor source and HLA disparity have consistently been found to be risk factors for the development of acute GVHD.12 This study demonstrates that 48.6% of the patients developed acute GVHD with Grade II being found in 54.5% of the cases. Some studies12,18,19 demonstrated similar incidences, however others2,10 found lower incidences, and Faraci et al.20 found an incidence of 75%. In this report, as well in Barker et al.17 and Hwang et al.,21 there was no difference in the incidence of acute GVHD between the BM and the UCB groups; however, Wang et al.22 reported that the incidence was lower in the UCB group.
The incidence of chronic GVHD ranges between 30% and 50% and thus is a major cause of non-relapse mortality and morbidity in long-term survivors. Chronic GVHD is associated with a graft-versus-leukemia effect (GVL) resulting in a decreased incidence of relapse.13 In this study, the incidence was 9.2% versus 25.0% as reported by Zecca et al.13 However in this study the follow-up was only up to 180 days after transplant. Similar to other reports,21,22 chronic GVHD occurred more in the BM group (p-value=0.007). Barker et al.17 reported no difference between the two groups.
Relapse occurred in 24% of the patients who received HSCT for malignant diseases with the incidence being similar in both groups. Wang et al.22 stated that relapse was lower in the UCB group. GVHD did not show a GVL effect, similar to the report by Lee et al.23; however Yi et al.24 believe that GVHD has an important protective role. The stage of the disease was important for prognosis in this study and in others.18,25
The estimated one-year OS after HSCT in the BM and the UCB groups were 58% and 52%, respectively (p-value=0.466). These data were found to be similar to the literature.3,6,17 In the multivariate analysis, the results associated with a significantly worse OS included higher age, non-use of ATG and GVHD. Petterson et al.6 reported that only patients with GVHD had worse survival, and Wagner et al.1 stated that HLA mismatch influenced survival too. Infection was the main cause of death as in other publications.2,25
ConclusionOur findings confirm that unrelated HSCT with BM and UCB results in similar complications and survival in the pediatric population. Therefore, UCB is a safe cell source for patients who do not have a sibling donor for HSCT.
Conflicts of interestThe authors declare no conflicts of interest.