Describe the clinical and laboratory characteristics and the transfusion strategy of patients at Hospital Israelita Albert Einstein with platelet refractoriness and identify their etiological characteristics. Standardize the platelet immunofluorescence technique by flow cytometry as a test for platelet compatibility in immune platelet refractoriness in transfusion support.
MethodsReview of medical records of refractory platelet patients followed at HIAE from January 2011 to May 2017. Clinical-demographic data, laboratory data and identification of the use of compatible genotyped platelets for patients in need of transfusion therapy were collected. The analyzed patients were classified according to the etiology of their platelet refractoriness. To standardize the FC-PIFT technique, blood group O platelets were incubated with serum from blood group AB donors and anti-IgG monoclonal antibody to determine the negative control. In order to verify the influence of the ABO system, monoclonal anti-IgG antibodies were incubated with blood group A or B platelets and with blood group O donor serum with isohemagglutinins below and above 1/64.
ResultsA total of 47 patients were evaluated, a 51% (24/47) preponderance of associated immune and non-immune factors (NIPR + IPR). The most common causes of NIPR + IPR were splenomegaly (54%) and the development of HLA antibodies (88%), consistent with the literature. For patients who required therapeutic transfusion, only a small portion received compatible genotyped platelets.
ConclusionAlthough 60% of patients could benefit from the therapeutic transfusion of genotyped platelets, only 10% were actually transfused with this type of blood component. This reaffirms the need for investments in a bank of genotyped platelet donors.
In the 1950s, platelet transfusion was successfully used to treat patients with hemorrhage.1 Since then, this procedure has been used more and more, mainly in patients with cancer, malignant hematological diseases and in hematopoietic stem cell transplantation.1 However, failure in platelet transfusion is a frequent phenomenon affecting 7 to 34% of oncological/hematological patients.2 One of the possible reasons for failures in platelet transfusion is the platelet refractoriness (PR), mainly in chronic transfusion support. Platelet refractoriness is defined as an inadequate well-documented increase after two transfusions with fresh platelets from ABO-compatible randomized donors.3,4
The documentation of the platelet increase can be performed through indexes, the most common of which are described in Table 1.
Non-immune and immune factors have been associated with platelet refractoriness. In PR patients with cancer or hematological diseases, non-immune factors were present in 72 to 82% of the cases and immune factors, in 25 to 39%.1 The non-immune factors present in patients which influence the response to platelet transfusion are splenomegaly, use of heparin, fever, use of amphotericin, bleeding and disseminated intravascular coagulation.5,6 Immune causes include alloimmunization to the human leukocyte antigen (HLA) and/or the human platelet antigen (HPA) due to previous exposures to transfusions, pregnancies and transplants, ABO incompatibility and platelet autoantibodies (autoantibodies to platelet glycoproteins). When ABO transfusion compatibility is not respected, the most frequent cause of PR5 is the presence of anti-HLA antibodies. The presence of anti-HLA antibodies is the most common cause of immune PR and was present in 90% of low-platelet transfusions in a study on patients with leukemia.4
Anti-HLA and/or anti-HPA antibodies can be identified through commercially available tests, such as the lymphocytotoxicity test, the platelet immunofluorescence test (PIFT) by microscopy or flow cytometry (CAPTURE-P®) and immobilization of platelet antigens by monoclonal antibodies (MAIPA).7 The possible strategies for the management of patients with immune PR consist in selecting HLA-compatible donors from a blood bank with genotyped platelets, identifying anti-HLA antibodies and avoiding donors with antigens that are recognized by these antibodies or performing a platelet crossmatch for the selection of compatible platelets.8
It is known that PR is associated with adverse events and increased hospital costs,9 with the identification of the main causes and the detection of the presence of alloimmunizations being of great importance for the proper management of patients in transfusion support.
ObjectiveThe present study aimed to analyze and describe the clinical and laboratory characteristics of PR patients treated at the Hospital Israelita Albert Einstein (HIAE) during January 2011 to May 2017, a population that has not yet been characterized in terms of platelet refractoriness. The Hospital Israelita Albert Einstein boasts 650 beds and approximately 1,000 transfusions of blood units per month. In addition, we sought to standardize the platelet immunofluorescence technique by flow cytometry (FC - PIFT) as a cross-platelet test in immune platelet refractoriness and to check the possibility of interference from ABO system antibodies (anti-A and anti- B) in determining the presence of anti-HLA or anti-HPA antibodies since there were no publications related to this subject in the literature review.
MethodsPopulationPatients diagnosed with platelet refractoriness treated at the Hospital Israelita Albert Einstein (HIAE) during the period from January 2011 to May 2017 were evaluated retrospectively and consecutively. From data obtained from the electronic medical record, clinical-demographic data (sex, age and underlying disease), platelet transfusion indication at the time of refractoriness and laboratory data (presence of anti-HLA and/or anti-HPA antibodies already performed in the institution's care routine) were collected. It was also identified which patients would have an indication for transfusion of compatible HLA or HPA platelets and to which of them it would be possible to apply this transfusion strategy. All patients received leukodepleted platelet units.
The study was approved by the Research Ethics Committee of Hospital Israelita Albert Einstein, Certificado de Apresentação de Apreciação Ética (CAAE) no. 65539717.6.0000.0071.
PR ClassificationThe analyzed patients were classified according to the etiology of their platelet refractoriness, being grouped as non-immune platelet refractoriness (NIPR), immune platelet refractoriness (IPR) and non-immune and immune platelet refractoriness (NIPR + IPR). The NIPR group consisted of those in which the presence of fever, splenomegaly, use of vancomycin, amphotericin and heparin were verified or who were undergoing chemotherapy and/or bone marrow transplantation; the IRP group, the presence of anti-HPA and / or anti-HLA antibodies were verified, and; the NIPR + IPR group, associated characteristics of the NIPR and IPR groups were verified.
Standardization of the immunofluorescence technique by flow cytometry (FC - PIFT)Determination of Negative ControlPlatelet samples from plateletpheresis products previously collected in less than 48 hours and adjusted for the 100,000/µL count in PBS/EDTA/BSA were used for the reactions. The volume of 50uL of platelets from the single blood group O blood donors was added to 50 µL of serum from the blood group AB blood donors. This suspension was incubated at 37°C for thirty minutes. After three consecutive washes, 10µL of the antibody (Goat F (ab ') 2 Fragment Anti-Human IgG (H + L) -PE / Beckman Coulter) was added in the 1:100 dilution and incubated for 30 minutes in the light. After washing, the cells were resuspended in 500 µL of PBS/EDTA/BSA. A total of 19 assays, using a combination of platelets and different donor serum, were performed to determine the negative control.
Values between one and two standard deviations above the mean fluorescence intensity of the negative control were considered inconclusive and values greater than or equal to two standard deviations of the mean fluorescence intensity (MFI) of the negative control were considered positive.
The reactions were read on a flow cytometer (Beckman Coulter), with a minimum of 30,000 acquisitions per reading considered.
Determination of AB blood group interferenceUsing the protocol described above, a total of 12 assays were performed, six in which platelets from blood group A or B were incubated with blood donors from blood group O with isohemagglutinin titers below 1/64 and the other 6 assays platelets from the group blood A or B, with blood group O blood donors with isohemagglutinin titers above 1/64. The titer of 1/64 was determined according to the previous routine of the transfusion service.
ResultsPopulationA total of 47 patients were evaluated, being 23 women and 24 men. The median age was 61 years (1 to 87 years).
The diagnosis was hematological in 42 cases and of these, 41 had oncological disorders and one had Glanzmann's thrombasthenia, a rare inherited bleeding disorder. Of the patients with oncohematological disorders, 24 underwent chemotherapy. The diagnosis was non-hematological in 5 patients and of these, 3 were diagnosed with liver cirrhosis, 1 used anticoagulant and had extensive hematoma in the thigh and 1 had testicular neoplasia of germ cells.
The clinical-demographic characteristics are described in Table 2.
- Characterization of patients in relation to sex, age and underlying disease.
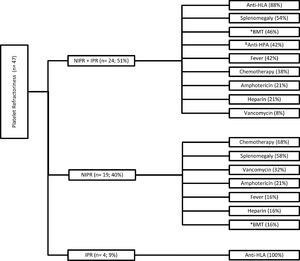
Regarding the etiological causes of platelet refractoriness, 51% (24/47) were due to associated non-immune and immune factors (NIPR + IPR), 40% (19/47) to non-immune factors (NIPR) and 9% (4/47) to immune factors (IPR). The details of the characteristics of platelet refractoriness due to etiological cause are described in Figure 1.
Patients who presented NIPR + IPR (n = 24) had an association of six etiological causes in 4% of the individuals in this group, 33% had four causal factors and 21% had two causal factors. In this group, the most prevalent etiological factors were the presence of anti-HLA antibodies (88%) and splenomegaly (54%). Patients with NIPR (n = 19) had an association of five etiological causes in 11% of the individuals in this group, 37% had three causal factors and 16% had only one causal factor. The most prevalent etiological factors in this group were chemotherapy (68%) and splenomegaly (58%). For patients who had NIPR (n = 4), all had only one causal factor involved, the development of anti-HLA antibodies.
A transfusion requirement was observed in 60% (51% of NIPR + IPR + 9% of IPR) of the patients at the time of refractoriness, but only in 10% was it possible to transfuse compatible genotyped platelets from the platelet donor bank with genotyping known to the HIAE.
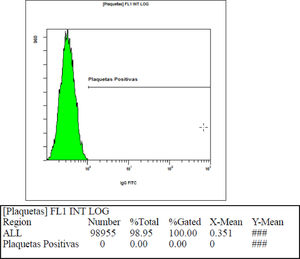
Standardization of the immunofluorescence technique by flow cytometry (FC - PIFT)Using blood group O platelets with serum from blood group AB donors, the mean fluorescence intensity value of the negative control found was 0.95 (two standard deviations = 1.46). Figure 2 shows the fluorescence intensity found in one of the samples used to calculate the negative control.
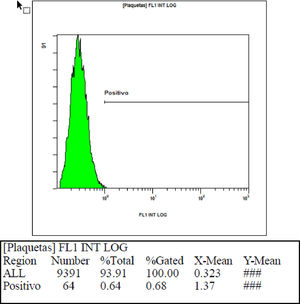
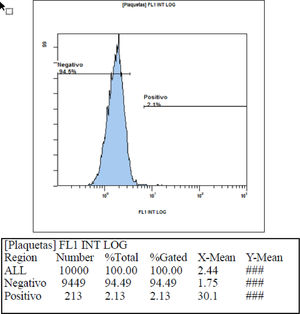
In checking the interference of the ABO system in the mean fluorescence intensity, we found that when blood group A or B platelets were incubated with blood group O blood donor serum with isohemagglutinin titers below 1/64, an average intensity value of 0.58 (two standard deviations = 0.69) was obtained, showing that these values are compatible with the previously found values of the negative control. When blood group A or B platelets were incubated with blood group O blood donor serum with isohemagglutinin titers above 1/64, a mean fluorescence intensity value of 1.51 (two standard deviations = 2.52) was obtained, showing that these mean values of intensity found are above the values found for the negative control. Table 3 shows the fluorescence intensity values found in each sample used to check the interference of the ABO system using serum with hemagglutinin titers below 1/64 and above 1/64. Figures 3 and 4, respectively, show an example of fluorescence intensity obtained using serum with hemagglutinin titers below 1/64 and above 1/64.
ABO system interference in the mean fluorescence intensity in flow cytometry.
In the study conducted on patients with thrombocytopenia associated with bone marrow transplant failure treated at the Peter MacCallum Cancer Institute, the main factors associated with low increment after one hour of platelet transfusion were bone marrow transplantation, administration of intravenous amphotericin B, alloimmunization to HLA antigens and a palpable spleen.10 In another study performed with patients at the Hospital Universitário La Fe, the risk factors associated with the low increase after fourteen hours of platelet transfusion were alloimmunization to HLA antigens, bone marrow transplantation and the amount of antibiotics used by the patients, the worst being increment rates that occurred with the use of amphotericin B, vancomycin and ciprofloxacin.11 In a study conducted with oncohematological patients from Minas Gerais, Brazil, 19% of the evaluated patients had PR, and the main factors involved were alloimmunization, splenomegaly, use of amphotericin B, use of vancomycin and fever.12 These are also factors predominantly found in the HIAE patients and in NIPR + IPR patients, 88% had anti-HLA antibodies, 54% splenomegaly, 46% had received BMT, 21% were using amphotericin B and 8% were using vancomycin. In NIPR patients, 58% had splenomegaly, 32% were using vancomycin, 21% were using amphotericin B and 16% received BMT.
In another study carried out in patients undergoing bone marrow transplantation at the Hospital São Camilo, 66.66% of refractory platelet patients used amphotericin B and 20% had fever and splenomegaly.13 In the HIAE patients, 42% of NIPR + IPR and 16% of NIPR had fever. In a study with patients in Porto Alegre, 67% had IPR, all of whom had anti-HLA antibodies and 19% also had anti-HPA antibodies.14 At the HIAE, of the patients who had only IPR, all developed anti-HLA antibodies and of the NIPR + IPR, 42% had anti-HPA antibodies concomitant with anti-HLA antibodies.
The prevalence of factors present in the HIAE platelet-refractory patients is in agreement with data found in the literature, although there is some difficulty in comparison, as in many studies patients are separated only by presenting non-immune factors or only presenting immune factors while there is predominance of associated immune and non-immune causes.
“In the present study, the prevalence of anti-HPA 1 were predominant among HPA antibodies specificities in immune refractory patients. This prevalence correlates with the international literature.15 However, the prevalence of anti-HPA 5 proves itself to be interesting. The Brazilian literature also highlights a different prevalence of anti-HPA 5 specificity in another cohort studied,16 a possible explanation to this finding being the heterogeneity of the Brazilian population due to ethnic admixture, as previously described in other platelet alloimmunization situations, suggesting a distinct characteristic of platelet alloimmunization in this population.” The HPA -5 system has also been shown to be an important risk factor for alloimmunization in the population of the Amazon.17
In the management of patients with PR, the treatment of non-immune causes has not changed significantly over the years and involves the identification of factors and their resolution.18 Therefore, it is important to know which factors are predominantly associated with non-immune PR, although it is difficult to resolve all the causes involved, since 48% of the patients had three or more factors involved in PR.
The management of patients with immune causes involves the identification of anti-HLA and/or anti-HPA antibodies and the transfusion of compatible genotyped platelets. Among the strategies available for compatible platelet transfusion, there are blood banks with HLA class I genotyping antigens from donors, as most alloimmunizations in immune PR, with rates ranging from 7% to 55%,19 are against HLA-A and HLA-B antigens and, after detection of immune refractoriness in the patient, a virtual cross between the recipient and the donor is performed for proper support with compatible platelets.20 There are several methods available for the identification of alloantibodies and cross-testing, the most used being the Luminex flow cytometry platform of single antigen class I for anti-HLA antibodies19,20 and the Monoclonal Antigen Immobilization Platelet (MAIPA) or the solid phase ELISA for anti-HPA antibodies.19
The present study also used flow cytometry to establish a cross-proof test in immune PR. In the standardization of the immunofluorescence methodology by flow cytometry (FC - PIFT) for the initial identification of anti-HLA and/or HPA antibodies, there was a need to establish two cutoff points for the mean fluorescence intensity value, one value in the presence of donor serum with anti-A and/or anti-B isohemagglutinins with titers less than 1/64 and another higher value in the presence of donor serum with anti-A and/or anti-B isohemagglutinins with higher titers than 1/64.
Among the various methods described in the literature for the identification of anti-HLA and/or anti-HPA antibodies, it is known that the flow cytometry (FC - PIFT) immunofluorescence test is comparatively easy and quick to perform and has good results with an accuracy of approximately 80%.7 However, there is no well-established information in the literature about the interference of anti-A and/or anti-B isohemagglutinin titers above 1/64 in the results when using the FC-PIFT test, which makes it difficult to compare the data found. It is important to pay attention to this interference, as false positive results can be incurred in the presence of anti-A and / or anti-B titers greater than 1/64.
Another technique for the management of patients with platelet refractoriness that is being developed and that is likely to be the future of blood banks is the production of induced pluripotent stem cells (iPSC) with the expression of silenced HLA. The perspective that is presented is of platelets with low immunogenicity, which prevents the allogeneic immune response even in non-immunosuppressed patients.21
ConclusionIt was observed that 60% (51% of NIPR + IPR + 9% of IPR) of the patients at the time of refractoriness would benefit from compatible genotyped platelet therapy, as immune causes have been identified in platelet refractoriness. However, only 10% of patients were actually transfused with this type of blood component. This difficulty in providing compatible components may be due to the genotypic variety found in our very mixed population and the limited number of genotyped donors (850) at our service. This highlights the need to invest in a donor bank with genotyped platelets, as this type of component can contribute to more effective transfusion strategies.
Conflicts of interestThe authors declare no conflicts of interest.