We evaluated different technological approaches and anti-D clones to propose the most appropriate serologic strategy in detecting the largest numbers of D variants in blood donors.
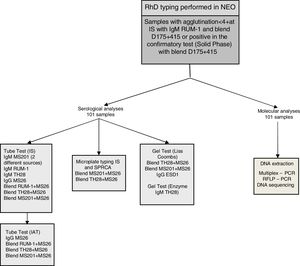
MethodsWe selected 101 samples from Brazilian blood donors with different expressions of D in our donor routine. The tests were performed in immediate spin (IS) with eleven commercially available anti-D reagents in a tube and microplate. The D confirmatory tests for the presence of weak D included the indirect antiglobulin test (IAT) in a tube, gel and solid-phase red blood cell adherence (SPRCA). All DNA samples were extracted from peripheral blood and the D variants were classified using different molecular assays.
ResultsThe RHD variants identified by molecular analysis included weak D types (1, 2, 3, 11 and 38) and partial Ds (DAR1.2, DAR1, DAR3.1, DAU0, DAU2, DAU4, DAU5, DAU6, DMH and DVII). The monoclonal-monoclonal blend RUM-1/MS26 was the best anti-D reagent used in detecting the D antigen in the IS phase in a tube, reacting with 83.2% of the D variants, while the anti-D blend D175 + 415 was the best monoclonal antibody (MoAb) used in a microplate to minimize the need for an IAT, reacting with 83.2% of the D variants. The D confirmatory tests using SPRCA showed a reactivity (3 - 4+) with 100% of the D variant samples tested.
ConclusionOur results show that, even using sensitive methods and MoAbs to ensure the accurate assignment of the D antigen, at least 17% of our donor samples need a confirmatory D test in order to avoid alloimmunization in D-negative patients.
The Rh system is the most clinically important blood group system after the ABO.1 Two highly homologous genes, RHD and RHCE, which are located on Chromosome 1 encode the RhD and RhCE proteins, respectively.2,3 The D antigen is the most important antigen of the Rh system because of its implication in the hemolytic transfusion reaction (HTR) and hemolytic disease of the fetus and newborn (HDFN). The D-negative phenotype is observed in 15–17% of Caucasians compared to 5% of Africans and less than 3% in Asian populations.4 In Caucasians, the D-negative phenotype results predominantly from the deletion of the RHD gene. In Africans, besides the RHD gene deletion and the inactive genes, the RHDΨ and the RHD-CE-Ds hybrid gene give rise to the loss of D expression.5 In South East Asian populations, 15–30% of D-negative individuals carry an RHD allele that encodes a protein with very weak expression of the D antigen, leading to the DEL phenotype,6,7 which can only be detected by adsorption-elution techniques.8
Although most individuals are either RhD-positive or RhD-negative, a plethora of variants of D have been described.9 The RHD gene is highly polymorphic and the D expression is caused by a large number of RHD alleles. A part of these alleles leads to a reduced or variable expression of D antigenic epitopes on the red cell surface.9 These variations in the RhD antigen structure result either in a partial or a weak D phenotype, leading to qualitative or quantitative changes in Rh protein expression, respectively.
Despite the fact that more sensitive monoclonal reagents have been produced, not all anti-D reagents detect the same partial or weak expression of the D antigen.10–12 Donors and patients with these atypical alleles may be mistyped by serology because many of these alleles do not react equally with all anti-D typing reagents.
Populations with African admixture, such as the Brazilian population, can present a high variety of RHD alleles.13 Considering this fact, the best strategy of D typing in the donor routine would be a suitable combination of anti-D reagents to identify weak D variants that could induce anti-D formation in D-negative recipients.
In this report, we evaluated different technological approaches and clones of anti-D to propose the most appropriate serologic strategy to identify weak D variants in the donor routine. We also performed molecular analyses to characterize the RHD alleles in order to know the RHD repertoire in this Brazilian population and to identify the RHD alleles undetected in serologic tests that could be responsible for transfusion-induced anti-D formation.
MethodsBlood samplesFrom November 2013 to August 2014, we selected a total of 101 blood donor samples from 123,936 samples. In the first month of this study, all donors with weak D expression were selected, which corresponded to 62 samples from a total of 12,560 donors (0.49%). The other 39 samples were selected based on discrepant results with previous donations or samples with very low antigen density. The RhD was typed in a microplate with two commercial anti-D monoclonal antibodies (MoAbs), RUM-1 and D175 + 415, using an automatic immunoassay analyzer (NEO®, Immucor, Norcross, GA, USA) that showed atypical D serologic typing (reactivity pattern <4+). This study was conducted in accordance with an institutional ethical review.
Serologic analysisOnce a discrepancy was noted with the automated assay NEO®, the red blood cells (RBCs) were further analyzed in immediate spin (IS) in a tube and microplate with eleven commercially available anti-D reagents from different sources (Lorne Laboratories, Berkshire, UK; Fresenius, São Paulo, Brazil; Bio-Rad, Lagoa Santa, Belo Horizonte, Brazil and Immucor, Norcross, GA, USA). Fig. 1 shows a flowchart of the study. The D confirmatory tests for the presence of weak D included the indirect antiglobulin test (IAT) in a tube and gel test for RBC samples with reactivity <2+ at immediate spin and in solid phase red cell adherence (SPRCA) for all RBC samples tested in a microplate. The protocols were performed according to the manufacturer instructions. The serologic reactivity was graded according to the degree of hemagglutination from 0 to 4+.
Molecular analysisThe DNA was extracted from whole blood using the QIAmp DNA Blood Mini-Kit (Qiagen, Valencia, CA), according to the manufacturer recommendations. The DNA concentration and purity were calculated by the measurement of the optical density at 260 and 280 nm using a NanoDrop 2000 Spectrophotometer (Thermal Cycler, Uniscience Inc., São Paulo, SP, Brazil) and DNA samples were kept at −20 °C. The molecular tests performed on all 101 samples with atypical results in serology included: a multiplex PCR, PCR-RFLP and Sanger sequencing, according to our approach previously described.14,15
ResultsMolecular findingsMolecular analyses confirmed that all RBC samples that showed atypical D serologic typing had altered RHD alleles, totalizing 15 different RHD alleles identified (Table 1). From 101 blood donor samples tested, 49 samples (48.5%) were molecularly characterized as partial D, 51 (50.5%), as weak D and 1, as weak/partial D (1%). The DAR1.2 was the most frequent allele found (29.7%), followed by weak D type 3 (20.8%), weak D type 38 (9.9%) and weak D type 1(8.9%). Less prevalent RHD alleles corresponded to 30.7% of all samples studied. Weak D types 1, 2 and 3 represented 37% of the RhD variants in this multiethnic population.
Molecular characterization of samples from 101 blood donors with weak or discrepant results in RhD typing.
| Partial RHD | N (49) | Weak RHD | N (51) | Weak/Partial RHD | N (1) |
|---|---|---|---|---|---|
| DAR1.2 | 31 | Weak D type 3 | 22 | Weak D type 2/ DAR1.2 | 1 |
| DAR1 | 5 | Weak D type 38 | 10 | ||
| DAR3.1 | 3 | Weak D type 1 | 9 | ||
| DAU4 | 3 | Weak D type 2 | 7 | ||
| DMH | 2 | Weak D type 11 | 3 | ||
| DAU0 | 1 | ||||
| DAU2 | 1 | ||||
| DAU5 | 1 | ||||
| DAU6 | 1 | ||||
| DVII | 1 |
Considering tube tests, our results showed a varied reactivity pattern among the several clones used, mainly in the IS phase, and these results are summarized in Table 2.
RhD variants and agglutination degree in IS test with several anti-D reagents.
| Lorne | Frese- | Bio-Rad | Immucor | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tube | nius Tube | Tube | Microplate | ||||||||||
| Molecular Analysis RHD | n | Agglutination Degree | IgM RUM1 | IgM MS201 | Blend RUM-1/MS-26 | IgM MS201 | IgM TH28 | Blend TH28 + MS26 | Blend MS201+ MS26 | IgM RUM-1 | Blend D175 + 415 | Blend MS201 + MS26 | Blend TH28 + MS26 |
| DAR1.2 | 30 | 0-1+ | 9 | 9 | 10 | 21 | 24 | 22 | 23 | 9 | 8 | 25 | 23 |
| 2-4+ | 21 | 21 | 20 | 9 | 6 | 8 | 7 | 21 | 22 | 5 | 7 | ||
| Weak D Type 3 | 21 | 0-1+ | 7 | 9 | 7 | 13 | 14 | 13 | 12 | 6 | 1 | 18 | 16 |
| 2-4+ | 14 | 12 | 14 | 8 | 7 | 8 | 9 | 15 | 20 | 3 | 5 | ||
| Weak D type 38 | 10 | 0-1+ | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 2-4+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Weak D Type 1 | 9 | 0-1+ | 6 | 6 | 6 | 7 | 6 | 6 | 6 | 5 | 3 | 5 | 5 |
| 2-4+ | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 4 | 6 | 4 | 4 | ||
| Weak D Type 2 | 7 | 0-1+ | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| 2-4+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| DAR1 | 5 | 0-1+ | 3 | 3 | 3 | 5 | 4 | 4 | 3 | 0 | 0 | 5 | 5 |
| 2-4+ | 2 | 2 | 2 | 0 | 1 | 1 | 2 | 5 | 5 | 0 | 0 | ||
| Weak D type 11 | 3 | 0-1+ | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 2-4+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| DAR3.1 | 3 | 0-1+ | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 2-4+ | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 2 | ||
| DAU4 | 3 | 0-1+ | 2 | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| 2-4+ | 1 | 3 | 1 | 2 | 3 | 2 | 2 | 3 | 3 | 3 | 2 | ||
| DAU0 | 1 | 0-1+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2-4+ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| DAU2 | 1 | 0-1+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2-4+ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| DAU5 | 1 | 0-1+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2-4+ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| DAU6 | 1 | 0-1+ | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 2-4+ | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | ||
| DMH | 1 | 0-1+ | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 2-4+ | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ||
| DVII | 1 | 0-1+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 2-4+ | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | ||
| Weak D type2/DAR1.2 | 1 | 0-1+ | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2-4+ | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Weak D type 3/RHDψ | 1 | 0-1+ | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| 2-4+ | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | ||
| DAR1.2/RHDψ | 1 | 0-1+ | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| 2-4+ | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | ||
| DMH/RHDψ | 1 | 0-1+ | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 2-4+ | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ||
The Lorne IgM and blend clones were more sensitive, when compared with the other clones analyzed. The RUM-1 monoclonal IgM and the monoclonal blend RUM-1/MS26 were the best monoclonal (MoAb) anti-D reagents used to detect the D antigen in the IS phase in a tube, reacting with 84.1% and 83.2% of the D variants, while the anti-D blend D175 + 415 was the best MoAb used in a microplate to minimize the need of an IAT on donor samples, also reacting with 83.2% of the D variants. These results can be observed in Tables 3 and 4.
Percentage of samples detected according to agglutination degree in IS tube method with IgM and blend anti-D clones.
| Source | Lorne | Fresenius | Bio-Rad | ||||
|---|---|---|---|---|---|---|---|
| Agglutination degree | IgMRUM1 | IgMMS201 | BlendRUM1+MS26 | IgMMS201 | IgMTH28 | BlendTH28+MS26 | BlendMS201+MS26 |
| % | % | % | % | % | % | % | |
| 3-4+ | 23.7 | 21.8 | 25.7 | 9.9 | 13.8 | 11.9 | 15.9 |
| 1-2+ | 46.5 | 52.5 | 44.6 | 46.5 | 40.6 | 41.6 | 45.5 |
| W* | 13.9 | 7.9 | 12.9 | 21.8 | 25.7 | 22.8 | 17.8 |
| 0 | 15.9 | 17.8 | 16.8 | 21.8 | 19.9 | 23.7 | 20.8 |
Percentage of samples detected according to agglutination degree and reaction score in IS microplate with blend anti-D clones.
| Source | Immucor | |||
|---|---|---|---|---|
| Agglutination degree Reaction score | IgM RUM-1 | Blend D175 + 415 | Blend MS201 + MS26 | Blend TH28 + MS26 |
| % | % | % | % | |
| 3-4 + 60-99 | 23.8 | 32.7 | 12.9 | 11.9 |
| 1-2 + 40-59 | 49.5 | 42.6 | 18.8 | 35.6 |
| W* 21-39 | 5.9 | 7.9 | 16.8 | 12.9 |
| 0 0-20 | 20.8 | 16.8 | 51.5 | 39.6 |
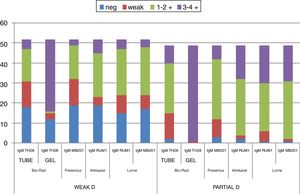
The monoclonal anti-D IgM RUM-1 and the blend RUM-1/MS-26 reagents were reactive with all DAR1.2, DAR1 and weak D Type 3 samples in a tube and most of them reacted 1+ to 2+, while the other anti-D reagents showed variations in the reactivity.
Weak D types 1 and 2 showed heterogeneity in their reactivity with the anti-D reagents in IS. The monoclonal anti-D IgM RUM-1 was the only reagent to give positive reactions with all weak D type 1 samples analyzed, while weak D type 2 samples had at least one IS-negative result with all clones tested. Nevertheless, all of these samples were detected by an IAT with all anti-D reagents tested. Similar results were obtained in a microplate with the anti-D IgM RUM-1 and blend D175 + 415 reagents, but this method was less sensitive with the other anti-D MoAbs used. All blood samples classified as DAR1.2, DAR1, weak D types 1, 2 and 3 reacted with all the anti-D monoclonals tested by gel. The DAR3.1, DAU0, DAU2, DAU5, DAU6, DVII and DMH were detected in IS with all reagents and methods and reacted strongly in IAT in a tube, gel and SPRCA.
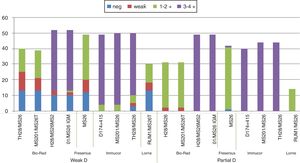
In relation to the tests performed with the gel technique, the D status was assigned as positive in 91 samples (90% of the total), with the blend clones TH28 + MS26 and MS201 + MS26 Bio-Rad in Liss/Coombs gel and 89 samples (88.1%) with the IgM TH28 Bio-Rad in NaCl/Enzyme gel. Of the 35 samples tested by ESD1, all of them showed positive results with scores similar to those of the other clones tested, but with improved performance in detection of the variant type 38.
Considering the clones tested with all samples: Blend TH28 + MS26, MS201 + MS26 and IgM TH28, only 30% of the weak D type 38 and none of the three Weak D type 11 samples were detected by an IAT in gel, while all these variants reacted strongly (3 - 4+) with the Immucor anti-D Blend D175 + 415 in SPRCA.
Overall, D confirmatory tests using gel cards showed agglutination reactions (3 - 4+) in 87.1% of the samples, detecting other variants, but serologic agglutination patterns were more consistent with the MoAbs in the solid phase, in which 100% of the samples showed strong positive reactivity (3 - 4+). The differences between clones and methodologies are demonstrated in Figs. 2 and 3.
Determining RhD antigen status in blood donors with reliable reagents and methods is essential to detect D variants in order to avoid alloimmunization in RhD-negative patients. However, the serologic distinction between D-positive and D-negative RBCs is not always straightforward in the case of D variants.16 Monoclonal anti-D reagents are evaluated for their ability to identify some D variants and not react with other variants.
Herein we have demonstrated the serologic RhD typing complexity to identify D variants, showing the differences of reactivity obtained with different anti-D reagents and methods. In the donor samples studied, five different weak D types (1, 2, 3, 11 and 38) and eleven partial D types (DAR1.2, DAR1, DAR3.1, DAU0, DAU2, DAU4, DAU5, DAU6, DMH and DVII) were identified by molecular analyses.
The RHD alleles are grouped into D clusters, some of which comprise many alleles. Among the weak D type 4 cluster, partial D DAR1.2 was the most prevalent partial D in our donors, corresponding to 30% of our samples. This finding is in agreement with the previously reported frequency of the DAR1.2 allele in Brazilian blood donors.17 We observed that the variant alleles from the weak D type 4 cluster were better reactive with an IAT with all anti-D monoclonals and methods used.
Among the DAU cluster, we found a small number of samples classified as DAU0, DAU2, DAU4, DAU5 and DAU6, and all of them were detected in IS, except DAU4, which was not detected by the MoAb anti-D IgM RUM-1, nor by the blend RUM-1/MS-26 reagents. Of these, only DAU0 was found in Caucasians, while the other four DAU alleles were observed in Africans.18
Among the most common weak D types, weak D types 1, 2 and 3 were found in 37% of our D variant blood donors. This finding is comparable to frequencies published for other donor populations from Southeast Brazil.13,17,19 but is in contrast with studies performed on Europeans, in whom these weak D types are common, encompassing more than 90% of all European weak D individuals.20,21 The weak D type 1 was better detected with the MoAb anti-D IgM RUM-1 and therefore, we can recommend this clone for detection of this weak D type in IS in a tube or microplate. The weak D type 2 showed a great variability in the reactivity in the IS phase with all the anti-D MoAbs used and, for this weak D type, we would recommend the indirect antiglobulin test in gel, or the SPRCA test. The weak D type 3 was the most prevalent weak D type found in our donor population, representing 21.7% of the samples and was reactive with most of the anti-D monoclonals employed.
By changing reagents and technological approaches, D antigen discrepancies between existing tests and historical records occur. When the automation using an immunoassay analyzer (NEO®, Immucor, Norcross, GA, USA) was introduced in our blood donor routine, D typing discrepancies were found with previous manually performed typing results. Most of them were related to weak D types 38 and 11, that have very low antigen density,21,22 which had been classified as D negative in previous donations. Weak D type 38 is predominant in the Portuguese population,23 but has also been found with a considerable frequency among Brazilians,19,22 and corresponded to 10% of our D variant samples. Considering only the 101 study samples, when we performed a look back, we observed that 7 of 10 donors classified as weak D type 38 were mistyped as D negative in previous donations. Likewise, all donors with weak D type 11 identified in this study were previously typed as D negative. Considering all mistyped donors, a total of 37 blood units typed as D negative were uncorrected in the previous donations. Costa et al.22 showed a similar scenario in 4 of 520 donors typed as RhD-negative, who were molecularly typed as weak D. Regarding these samples, no discrepancies involving other RhD variants were observed.
Serologic discrepancies caused by D variants are more likely to be resolved by genotyping, but this strategy cannot be applied routinely at all centers due to the cost and feasibility. The reactivity of the monoclonal anti-D reagents depends on the concentration and avidity of the antibodies. In fact, the number of antigenic sites, accessibility of an epitope, the presentation, the concentrations and the class of immunoglobulin can affect the reactions of weak and partial RhD antigens with monoclonal reagents.24 The choice of the reagents and methodology requires special attention, mainly in a mixed population with the influence of Caucasian, Indian and African ethnicities, as are the Brazilians, leading to a diversity in D variants, in addition to an atypical heterozygous composition or the presence of new alleles.
In this study, the clones IgM RUM-1 and blend RUM-1/MS26 had better performance in detecting D variants in the IS tube tests, while the blend D175 + 415 showed better reactivity in microplates. Even considering the fact that the ESD1 was introduced later into the study, and we could not test it with all variants, including the variant weak D type 11, this clone was shown to be a good option for the IAT gel. When we compared ESD1 with the other Bio-Rad clones, we found a higher reactivity pattern, especially regarding the variants of low antigenic density. The proposed clones for each method described in this work are represented in Fig. 4.
Regarding the technological approaches used, all of them have positive and negative points. Although the tube test has a short incubation, it was shown to be less sensitive in detecting D variants, requiring a greater RhD work-up. The gel test is more sensitive and could be used in an automated process. The solid phase is used as a confirmatory test in the automated analyzer NEO® and was the only methodology able to detect all the D variants identified, including the weak D types with very low density, such as the weak D types 38 and 11, that express less than 200 D antigen sites.
Success in donor D typing depends on the anti-D reagent used and on the exact conditions of the methods. Our results show a great variation in the methods and reagents in detecting D variants in Brazilian blood donors and, even using sensitive methods and different MoAb anti-D reagents to ensure the accurate assignment of the D antigen, at least 17% of the donor samples would need a confirmatory D test in order to avoid alloimmunization in D-negative patients. Sensitive confirmatory tests are indispensable in the detection of RhD variants in blood donors, particularly those with low antigenic density, and may not be detected, depending on the techniques and reagents used. This study allowed us to compare and determine the capacity and limitations of different technological approaches and reagents in identifying the most frequent D variants in a population with a high rate of miscegenation, particularly in individuals with weakened D expression.
Conflicts of InterestThe authors declare no conflicts of interest.