To evaluate the efficacy and safety of romiplostim (thrombopoietin-receptor agonist) in the treatment of pediatric immune thrombocytopenia (ITP).
MethodsSearches were conducted in MEDLINE, EMBASE, LILACS, Cochrane Central Register of Controlled Trials and ClinicalTrials.gov (from January 2011 to August 2021). Randomized controlled trials (RCTs), double-blind, comparing romiplostim with a placebo in pediatric persistent or chronic ITP were included. The primary outcome was the overall response rate (platelets ≥ 50 × 109/L) in the absence of rescue therapy for at least two consecutive weeks. The secondary endpoints were the minimization of clinically significant bleeding and the necessity for rescue treatments and the maximization of safety (incidence of overall adverse events) and durable response (maintaining platelet counts for at least twelve weeks).
ResultsTwo double-blind randomized placebo-controlled trials (84 participants) were included in this systematic review. Our data showed that, compared to the placebo group, the proportion of patients achieving durable platelet response was significantly higher in the romiplostim group (p = 0.003, RR = 6.34, 95%CI = 1.89 - 21.23), as was the overall response in the romiplostim group (p = 0.002, RR = 3.62, 95%CI = 1.63 - 8.03). Significant bleeding incidents (p = 0.49), overall adverse events (p = 0.71) and the need for rescue treatment (p = 0.13) were not statistically different between the romiplostim and placebo groups.
ConclusionsRomiplostim might improve both durable and overall platelet response in children and adolescents with ITP, compared to a placebo. More clinical trials are needed to evaluate the efficacy and safety of romiplostim and to compare it with other second-line treatments that are being used in pediatric ITP.
Immune thrombocytopenia (ITP) is an acquired immune disease characterized by a transient or persistent decrease in the platelet count and risk of bleeding, depending upon the degree of thrombocytopenia.1
A platelet count of less than 100 × 109/L has been established as the threshold for diagnosis. The International Working Group defines ITP as newly diagnosed (from diagnosis to 3 months), persistent (3 to 12 months from diagnosis), or chronic (lasting for more than 12 months). ITP may occur in isolation (primary) or in association with other disorders (secondary), such as infections, other autoimmune disorders (systemic lupus erythematosus, antiphospholipid syndrome), drugs and malignancy.2
The pathophysiology is characterized by antiplatelet autoantibodies causing premature removal of platelets from circulation by macrophages in the reticuloendothelial system and suppression of megakaryocyte production, maturation and platelet release.3–6 The increased platelet destruction and reduced platelet production help explain why different drug strategies are more effective in some patients than in others.7
Thrombopoietin (TPO) is a potent megakaryocyte colony-stimulating factor and, along with other cytokines, increases the size and number of marrow megakaryocytes and circulating platelets.8
Romiplostim is a thrombopoiesis-stimulating agent, composed of four identical peptides that bind to the thrombopoietin receptor c-MpL fused to an Fc fragment to prolong its half-life. It is administered weekly as a subcutaneous injection (1 – 10 µg/kg).8 It was approved by the Food and Drug Administration (FDA) in 2018 for pediatric patients who have had an insufficient response to corticosteroids, immunoglobulins or splenectomy, aged one year and older, with ITP for at least 6 months.9 The result is the multiplication, growth and maturation of megakaryocyte cells and, ultimately, platelet production.8 Romiplostim side effects in pediatric patients include contusion, upper respiratory tract infection and oropharyngeal pain.9
The aim of this study was to assess the short-term efficacy and safety of romiplostim therapy in children and adolescents with ITP refractory to standard medical therapy. This was possible through a systematic and in-depth review and statistical synthesis of appropriate data from the chosen studies.
Material and methodsThis study was conducted according to Preferred Reporting Items for a Systematic Review and Meta-analysis (PRISMA). We registered in PROSPERO International Prospective Register of Systematic Review (PROSPERO 2021: CRD42021274101).
MEDLINE (Medical Literature Analysis and Retrieval System Online/PubMed), EMBASE, LILACS (Latin American and Caribbean Literature in Health Sciences), Cochrane Central Register of Controlled Trials (CENTRAL) published in the Cochran Library and ClinicalTrials.gov were searched from January 2011 to August 2021. The search strategies are outlined in Table 1.
Search strategies.
MeSH: Medical Subject Headings; Emtree: Excerpta Medica Thesaurus; DeCS: Descriptors in Health Sciences.
We also searched conference proceedings of the American Society of Hematology and European Hematology Association and conducted manual searches in the references lists of included studies and in the gray literature (e.g., Google Scholar).
Titles and abstracts of retrieved articles were screened by the electronic search strategies for analysis. The three authors then independently evaluated the full-text versions of each potentially relevant study for inclusion in the systematic review and for detailed review. Disagreements between authors were resolved through discussion. If necessary, arbitration was provided by the senior author. We included studies published in English, Spanish and Portuguese.
All included studies met the following criteria: double-blind randomized controlled studies; participants were children and adolescents (≤ 18 years) with ITP lasting for six months or longer; the intervention was romiplostim, irrespective of dosage and schedule, and; the comparison was a placebo.
The exclusion criteria included: studies including both children and adults, if the data of children could not be extracted separately, studies including patients with Evans syndrome or secondary ITP and studies where other medication was given prior to four weeks before administrating romiplostim
The primary endpoint was the overall response rate (platelets ≥ 50 × 109/L), in the absence of rescue therapy for at least two consecutive weeks. The secondary endpoints were the reduction in the proportion of patients needing rescue treatment (e.g., new drugs, increased dose of a concomitant drug from baseline, platelet transfusion or splenectomy) for immediate risk or treatment failure and overall or clinically significant bleeding, according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0,10 and the enhancement of safety (reduction in the incidence of overall adverse events) and durable response (maintaining platelet counts for at least twelve weeks).
The following data were independently extracted by two researchers: general study details (authors, year of publication and country of origin), study design and use of control, sample size randomized into each group, dose and schedule of romiplostim; the outcomes of each study; numerical data for assessment of included outcomes, and; sources of funding.
We evaluated the risk of bias in individual studies using the Cochrane Risk of Bias Tool. The assessment was performed using the Review Manager Software version 5.4 (RevMan 5.4). If studies were homogeneous in terms of design and comparator, we conducted meta-analysis. Dichotomous data were determined by using the risk ratio (RR) with the 95% confidence interval (CI). We used the Chi-square test (significance level: 0.1) and the I2 test to define heterogeneity. A value for I2 ≥ 50% or p < 0.1 was used to denote significant heterogeneity. We used a fixed-effects model to synthesize data when heterogeneity was not significant (I2 < 50%).
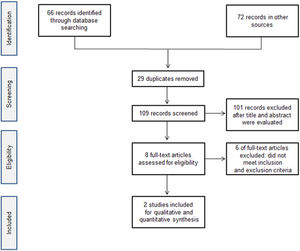
ResultsA total of 138 citations were obtained from the literature search and the selection process is shown in Figure 1. Two double-blind randomized, placebo-controlled studies (84 participants) [11, 12] were included in this systematic review. The studies were multicenter, from different countries (United States, Spain, Australia and Canada). The trials included mainly studied Caucasians and, in a lower percentage, African-Americans and other ethnicities. All patients were aged 1 – 17 years, and with disease duration over 6 months. Fifty-one percent of patients were male. Ten patients had undergone splenectomy (seven randomized to romiplostim and two to placebo) and 32 (38%) had received more than three previous immune thrombocytopenia treatments. All the included studies had patients with a mean platelet count under 30×109/L. Baseline patient characteristics and study characteristics are shown in Table 2 and Table 3.
Characteristics of included studies.
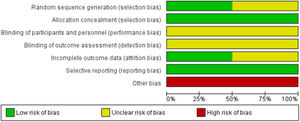
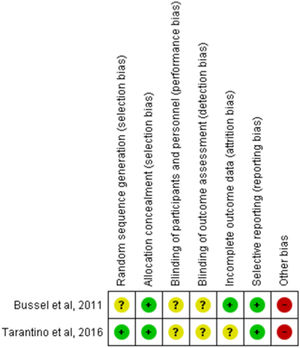
The risk of bias is graphically summarized in Figure 2 and 3. One study [11] had unclear risk of selection bias for central randomization because the method of randomization concealment was not reported. Both studies had unclear risk of performance and detection bias because the blinding of participants, clinicians, data collectors, outcome adjudicators and data analysts were not well described in each paper. One study [12] had unclear risk of attrition bias because one participant was excluded without explanation. We characterized as having a high risk for bias the two studies that were supported by the pharmaceutical industry.
After our statistical synthesis, we evaluated the number of patients that achieved a post-treatment platelet count equal to or over 50×109/L, without the need for rescue treatment for at least two weeks. One study [11] reported that 15 of the 17 (88%) patients in the romiplostim group achieved a platelet count response for two consecutive weeks within 12 weeks, while one of the five (20%) patients in the placebo group reached this criterion. The second study [12] reported that 30 of 42 (71%) patients in the romiplostim group and 4 of 19 (21%) patients in the control group had overall platelet response and achieved platelet response during weeks 2 – 25. We conducted a meta-analysis to picture those results and to achieve an approximate estimate of RR, even if primary studies had a different definition and follow-up of the overall response. The pooled result showed that patients who received romiplostim were almost 3.6 times more probable to achieve the primary target, when compared to patients who received the placebo (p = 0.002, RR = 3.62, 95%CI = 1.63 to 8.03, I2 = 0%; Figure 4).
Both studies compared the need for rescue treatment between the romiplostim and placebo groups. The pooled result (fixed effects, I2 = 0%) showed that there was no significant difference between the two groups (p = 0.13, RR = 0.47, 95%CI = 0.18 – 1.24; Figure 5).
The number of clinically significant bleeding incidents (CTCAE ≥ 2: moderate adverse event with medical intervention indicated) was not statistically different between the romiplostim and placebo groups (p = 0.49, RR = 1.82, 95%CI = 0.33 – 9.96; Figure 6).
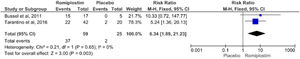
Our findings showed that patients receiving romiplostim achieved durable platelet response significantly higher than those receiving the placebo (p = 0.003, RR = 6.34, 95%CI = 1.89 – 21.23; Figure 7).
Both studies reported overall adverse events and our analysis indicated that there was no statistically significant difference between the two groups (romiplostim and placebo), (p = 0.71, RR = 1.02, 95%CI = 0.92 - 1.14; Figure 8).
The most common adverse events associated with romiplostim were contusion (18% to 50%), epistaxis (35% to 48%), headache (35% to 43%), upper respiratory tract infection (12% to 38%), oropharyngeal pain (24% to 26%), vomiting (12% to 26%) and fever (21% to 24%). [11, 12] None of the patients stopped therapy because of adverse events.
DiscussionChildren and adolescents with chronic ITP and pronounced thrombocytopenia who are at risk for major bleeding or health-related quality of life implications are treated with second-line therapy.
Unfortunately, there is no established second-line therapy algorithm to follow. Selecting a specific treatment is challenging, as long-term corticosteroid regimens have toxicity and many patients require a high dose; splenectomy, with response rates of approximately 70% in children carries a risk of sepsis,13 that may be increased if an underlying immune deficiency disorder contributes to ITP; rituximab therapy is reported to long-term remission in only 20 to 25% of patients14; danazol may impact sexual development and final height in pre-pubertal children, and; cyclosporine, mycophenolate mofetil and azathioprine are not well defined for pediatric patients with chronic ITP.14 More recently, two thrombopoietin-receptor agonists (eltrombopag, 2015 and romiplostim, 2018) have been approved for the treatment of children with ITP.9,15
Given this diversity of second-line treatments, prescribers need to understand the effect of each treatment to select the best for an individual patient.
This systematic review incorporating a meta-analysis summarized the efficacy and safety of romiplostim in children and adolescents with ITP. Our study suggests that the use of romiplostim may improve the durable and overall platelet response, compared to the placebo. It has been shown that romiplostim might not reduce bleeding events (moderate and severe bleeding) and that it may not reduce the need for rescue treatment in children with ITP.
A systematic review on the efficacy of TPO-receptor agonists in children found that romiplostim significantly improved overall platelet response (p = 0.0001, RR = 5.05, 95%CI = 2.21 – 11.53) and durable response (52% of patients), compared to the placebo.16 Our results are different and this could be related to the fact that Zhang et al.16 included one single-blind randomized, placebo-controlled trial in their meta-analysis. It was not possible to analyze the differential platelet responses based on stratification by age because of the small patient numbers and the studies not being designed to examine a differential response between the splenectomized and non-splenectomized patients. In addition, clinical studies on pediatric patients did not evaluate the effects of romiplostim, compared to the standard care or other second-line treatments.
Pediatric patients treated with romiplostim may also present bleeding events with medical intervention indicated. In the pooled analysis, combined data from pediatric patients across five clinical trials of romiplostim (two completed double-blind, placebo-controlled trials, two completed open-label extensions and one ongoing open-label trial) approximately 10% of patients presented severe bleeding.17 Our study showed similar results in the romiplostim arm and 4.1% in the placebo arm. The number of clinically significant bleeding incidents and minor bleedings were not statistically different between romiplostim and placebo (p = 0.49, RR = 1.82, 95%CI = 0.33 – 9.96). It could be related to the low frequency of bleeding in both groups. These results should be interpreted with caution due to the small sample size and both studies used the bleeding scale based on the standard CTCAE grading system, that was not specific to children or ITP.
Rescue treatment was administered to 11.8% and 25% of patients on romiplostim and placebo, respectively, and the pooled result showed that there was no significant difference between two groups (p = 0.13, RR = 0.47, 95%CI = 0.18 – 1.24). Although these results are in disagreement with previous studies involving children and adults,18,19 these findings are consistent with clinically significant bleeding incidents, as discussed above.
The overall adverse events were similar to those reported in previous studies and included headache, fatigue, epistaxis, insomnia, nausea, diarrhea, dyspepsia, arthralgia, muscle pain, complications related to site of injections and flu-like manifestations.8
This systematic review has some limitations. We only included double-blind, randomized, placebo-controlled studies in pediatric patients; the results may not have good generalizability and the studies included the small sample size. Both studies were not sensitive to find rare adverse events related to the drug, as the sample size was small. It was difficult to combine some numerical results to produce a rigorous meta-analysis, as the studies used different measures in their analyses or different operational definitions. In addition, bias may have occurred related to the fact that both clinical research studies were funded by a pharmaceutical company.
ConclusionRomiplostim might improve both durable and overall platelet response in children and adolescents with ITP, compared to the placebo. However, no statistical differences in significant bleeding, adverse events and necessity for rescue treatment were detected between the two groups. Considering the limitations of the studies included, more clinical trials with larger patient samples are needed to evaluate the efficacy and safety of romiplostim and to compare it with other second-line treatments that are being used in pediatric ITP.