Although it is an essential component of the treatment of acute lymphoid leukemia in children, asparaginase causes adverse reactions that sometimes make it impossible to use it fully. Hypersensitivity reactions are the most frequent and may lead to early discontinuation of treatment. The present study aimed to investigate suspicions of adverse reactions during the infusion of asparaginase in a pediatric cohort.
MethodsA retrospective observational study was carried out at a university pediatric institute in the state of Rio de Janeiro. Information regarding clinical features and characteristics of adverse reactions was collected from hospital medical records. Suspicions of adverse reactions were classified regarding causality and severity.
ResultsSeventy-three suspicions of adverse reactions were recorded during asparaginase infusion in 72 children in the study period. Allergic hypersensitivity reactions were suspected in 60.5% of the cases. Of these, 25% of the reactions occurred during induction and 61.1% in concomitant use with vincristine, findings that diverge from other studies. High-risk classification and younger age were considered risk factors for these reactions. A total of 72.4% of the reactions were classified as grade 1 or 2, which suggest that not all are related to antibody formation; this highlights the importance of differential diagnosis with other reactions, such as non-allergic hypersensitivity and hyperammonemia.
ConclusionThe implementation of the differential diagnosis of reactions related to infusion of asparaginase with ammonia dosage and classification of the grade of reactions is crucial to facilitate the identification and proper management of each type of reaction.
Cancer is the main cause of death due to illness in the age group of 0–19 years in Brazil. In recent years there has been a progressive and linear increase in the number of pediatric cases, especially of acute lymphoblastic leukemia (ALL),1 which is the most common type in childhood. Treatment of ALL includes the use of the bacterial enzyme asparaginase because of its ability to hydrolyze the amino acid l-asparagine in aspartic acid and ammonia, thus affecting leukemic blasts, which are generally incapable of producing asparagine by their own metabolism, unlike normal cells, which have asparagine synthetase. Asparagine depletion causes disruption of the functioning of blasts and consequently cell death.2
Asparaginase was incorporated into the treatment of ALL in the 1970s as an essential component of induction regimens and consolidation of remission in pediatric ALL.3 Three enzyme preparations are used: (i) native l-asparaginase derived from Escherichia coli (EcA) or (ii) in the pegylated form (EcPA) and (iii) isolated l-asparaginase from Erwinia chrysanthemi (ErA).4 The main limitation of its use is the occurrence of adverse reactions that can provoke harmful, unintended responses, even with adequate doses. Some of these reactions, such as hypersensitivity reactions and hyperammonemia, can occur during asparaginase infusion.5
EcA can be administered intravenously (IV) or intramuscularly (IM). The IV route allows interruption of the infusion in case of anaphylactic reactions. This pathway is the least sensitizing among the parenteral routes, although with a higher risk of serious anaphylactic reactions.6 In addition, IM administration causes pain, may require multiple injections for high-dose administration and exposes the patient to the full dose, even with severe reactions. Recent studies have shown comparable incidence of hypersensitivity reactions in both routes.5
Hypersensitivity reactions are considered unpredictable and not related to dose.7,8 Among these, those that are triggered by an immunological mechanism and which may cause anaphylaxis are considered allergic.6,9
Allergic hypersensitivity reactions impair the continuity of treatment with the type of l-asparaginase adopted, since inactivation of the enzyme may occur due to the formation of anti-asparaginase immunoglobulin IgG and IgE antibodies, making its use risky and ineffective.10,11
Some factors are related to a greater chance of developing hypersensitivity reactions such as the protocol phase (post-induction phases), route of administration, dose interval, type of l-asparaginase and concomitant use of drugs.2,4,10,12,13
Early discontinuation of treatment with the enzyme is common,14 especially in countries such as Brazil, where only the conventional EcA has been registered in the national health surveillance agency. Such an interruption may lead to inferior results when compared to patients who can use all the prescribed doses.4
The present study aimed at the analysis of reactions related to EcA infusions in a cohort of children with ALL treated over a period of ten years.
MethodsA longitudinal study was conducted with retrospective data collected on ALL treatment (in the phases of induction, consolidation and remission re-induction) in a pediatric university hospital located in the state of Rio de Janeiro.
Patients of up to 12 years of age, diagnosed with ALL in the period between January 2005 and December 2014 and treated with EcA according to the German protocol ALL-BFM-IC,11,15 were identified through the high-complexity treatment authorization lists, which allow the identification of procedures, such as chemotherapy, according to the individual and disease. This was supplemented by the hospital data records system.16 Infants treated with the INTERFANT protocol, patients on relapse treatment, as well as transferred patients who started or terminated treatment at another institution were excluded from the study.
Data were collected from specially designed treatment protocols used to register detailed information about the disease stage, the chemotherapy actually applied and complications, and the outcomes. The complete medical records were consulted when information was missing about the signs and symptoms of the reactions in these records.
The data related to the characterization of the patient (age at diagnosis and sex), leukemia (type of ALL, risk classification), treatment (treatment phase, prescribed medication, posology, route of administration) and description of suspicions of adverse reactions to EcA13,17 were collected on a specific form designed for this study and later organized on spreadsheets of the Microsoft Excel® program.
The causality of the adverse reaction was analyzed using Naranjo's algorithm.18 This tool classifies the possibility of the reaction being related to the drug, based mainly on the temporality of the event, history of the patient and evidence about the action of the drug. This algorithm, recommended by the World Health Organization (WHO) and the Ministry of Health,19 is based on the score obtained from ten questions related to the suspicion and is used to classify the reaction as dubious, possible, probable and defined.20 Severity analysis considered the description of signs and symptoms taking into account the common terminology criteria for adverse events (CTCAE v3.0)21 for allergic reactions/hypersensitivity.
The exploratory analysis of the data consisted in the calculation of measures of central tendencies (mean and median), dispersion (standard deviation) and proportions.
The analysis of adverse reactions related to the infusion demanded a review of evidence on the effectiveness and safety of EcA. The mechanisms related to allergic and non-allergic hypersensitivity in face of the occurrence of hyperammonemia, as well as aspects related to drug interaction with vincristine highlighted in the therapeutic scheme used in the studied institution were particularly important for the formulation of hypotheses related to causality and type of reaction presented during the intravenous infusion of EcA.
The binomial logistic regression model was used to calculate the univariate odds ratio of each variable selected as a possible risk factor for the development of hypersensitivity reactions from its biological plausibility in isolation. For multivariate analysis, variables with p-values <0.20 in the previous stage and after collinearity analysis were considered potential predictive variables to be included in the modeling. The stepwise method with backward elimination was used to select the parsimonious predictive model, using the likelihood ratio for its selection. All these analyzes were performed in the SPSS® program.
The research project was submitted to, registered in and approved by the Research Ethics Committee of the Institution (#1.218.087).
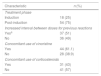
ResultsCase-by-caseOne hundred and twenty-five patients with ALL were treated during the study period. Of these, six cases fulfilled the exclusion criteria. The mean and median ages of the investigated group were 5.1 (±2.97) and four years, respectively. Table 1 shows the characterization of the patients.
Clinical characteristics of patients and suspected adverse reaction (n=119).
| Clinical characteristic | n (%) |
|---|---|
| Sex | |
| Male | 67 (56.3) |
| Female | 52 (43.7) |
| Type of ALL | |
| BCP-LLA | 101 (84.9) |
| T-LLA | 18 (15.1) |
| Risk classification | |
| Standard | 27 (22.7) |
| Medium | 43 (36.1) |
| High | 49 (41.2) |
| SRA during the asparaginase infusion | |
| Yes | 72 (60.5) |
| No | 47 (39.5) |
BCP-LLA: acute lymphoblastic leukemia of B-cell precursors; T-ALL: acute T-cell lymphoblastic leukemia; SRA: suspected adverse reaction.
All reactions related to EcA infusion were described as allergic in the medical records. All patients who presented reactions had their treatment interrupted. In only one case the reaction occurred twice, with different signs and symptoms; treatment was discontinued after the second reaction, thus 73 reactions are reported in 72 individuals.
Table 2 shows the exploratory analysis of the characteristics related to the treatment at the time of the appearance of the reaction that are pointed out in the literature as risk factors for allergic hypersensitivity. Different types of asparaginase and administration routes could not be analyzed because this is an observational study in which only EcA was used, and always by an IV route.
Descriptive analysis of characteristics related to the treatment at the time of reaction (n=73).
| Characteristic | n (%) |
|---|---|
| Treatment phase | |
| Induction | 18 (25) |
| Post-induction | 54 (75) |
| Increased interval between doses for previous reactions | |
| Yesa | 37 (51) |
| No | 36 (49) |
| Concomitant use of vincristine | |
| Yes | 44 (61.1) |
| No | 28 (38.9) |
| Concomitant use of corticosteroids | |
| Yes | 31 (43) |
| No | 41 (57) |
After applying the Naranjo's algorithm for causality analysis, 34 suspected adverse reactions were classified as possible and 39 as probable.
Only 29 cases (40%) could be classified according to severity (Table 3). The other suspicions of hypersensitivity reactions had no description of signs and symptoms in the medical records and were denominated only as ‘allergy’.
Description and classification of reactions regarding the severity (n=29).
| Grade | Stage of treatment | Signs and symptoms | n |
|---|---|---|---|
| 1 | Induction | Mild urticaria | 1 |
| Chest pain; cough | 1 | ||
| Re-induction | Vomiting, rash, and abdominal pain | 1 | |
| Abdominal pain; distension; agitation; tachycardia | 1 | ||
| Urticaria; cough; vomiting | 1 | ||
| Hyperemia on face; thoracic pain | 1 | ||
| 2 | Induction | Dyspnea, facial flushing, abdominal pain | 1 |
| Abdominal pain; difficulty in breathing, facial hyperemia; cougha | 1 | ||
| Rash exanthematic; tachypnea; Coughb | 1 | ||
| Urticaria; difficulty breathing | 1 | ||
| Consolidation | Cyanosis consolidation; plates; Pruritus; vomit | 1 | |
| Pruritus; urticaria | 1 | ||
| Cutaneous hyperemia; cough | 1 | ||
| Respiratory distress; cough | 1 | ||
| Vomiting; tachycardia; rash; difficulty breathing | 1 | ||
| Re-induction | Generalized pruritus re-induction; blush; cough | 1 | |
| Abdominal pain; rash; urticaria | 1 | ||
| Respiratory distress; cough | 1 | ||
| Pruritus of face; odonophagia | 1 | ||
| Respiratory distress; facial flushing; cough | 2 | ||
| 3 | Induction | Wheezing, cough and conjunctival hyperemia | 1 |
| Consolidation | Edema; cyanosis labialis; skin rash; cough | 1 | |
| Edema; rash; trembling; chill and fever | 1 | ||
| Re-induction | Abdominal pain; edema of lips; dry cough | 1 | |
| Bronchospasm; rash on hands and feet | 1 | ||
| 4 | Re-induction | Respiration respiratory difficulty; anaphylaxis; cough | 3 |
Among the suspicions of hypersensitivity that could be classified, there was a predominance of grade 1 and 2 reactions (72.4%). It was observed, however, that some signs and symptoms described in cases of less severity, such as respiratory distress, vomiting, rash and agitation,12 are also common to increases in serum ammonia level after the administration of asparaginase and 13 patients had such symptoms.
Risk factors for the development of reactions related to asparaginase infusion were analyzed and it was identified that gender, type of ALL and previous hypofibrinogenemia were not statistically significant. Risk and age classification were statistically significant (Tables 4 and 5) and non-collinear. In a multivariate analysis, the high-risk classification was a risk factor for the development of these reactions (odds ratio: 2.801) with age proving to be a protective factor (0.883 for each year of life).
Risk factors for reactions related to asparaginase infusion.
| Reaction during asparaginase infusion | |||||
|---|---|---|---|---|---|
| Risk factor | Yes | No | Odd ratio | 95% confidence interval | p-value |
| Sex | |||||
| Female | 33 | 19 | 1.247 | 0.592–2.625 | 0.577 |
| Male | 39 | 28 | |||
| Type of ALL | |||||
| BCP-ALL | 63 | 38 | 1.658 | 0.605–4.542 | 0.433 |
| T-ALL | 9 | 9 | |||
| Previous hypofibrinogenemia | |||||
| Yes | 36 | 29 | 0.621 | 0.294–1.311 | 0.254 |
| No | 36 | 18 | |||
| Risk classification | |||||
| High risk | 36 | 13 | 2.615 | 1.189–2.755 | 0.021 |
| Not high risk | 36 | 34 | |||
| Age | 0.873a | 0.767–0.995 | 0.041 | ||
| SC | 0.351b | 0.080–1.547 | 0.167 | ||
BCP-LLA: acute lymphoblastic leukemia of B-cell precursors; T-ALL: acute T-cell lymphoblastic leukemia.
The distribution of sex and of type of ALL of this population were similar to other studies, with a discrete predominance of cases in boys and with approximately 85% of ALL being cases of B-cell precursors.4,12,13 The majority (41.2%) were high-risk patients. This profile differs considerably from that observed in the German study that was the basis of the ALL-BFM-IC 2002 protocol, in which only 17% were high-risk cases and there was a higher number of intermediate-risk patients (52.4%).13
Another relevant aspect was the occurrence of reactions (25% of the cases) in the induction phase. Allergic hypersensitivity reactions are predominant in the post-induction phases.10 Woo et al. reported an incidence of only 12% of hypersensitivity reactions in the induction phase.22
Although the frequency of hypersensitivity reactions in the literature is very variable (frequencies from 0% to 76%, with an average of 30% for patients using asparaginase derived from E. coli7,10), the percentage observed in the present study (60.5%) was high, a condition aggravated by the lack of alternative types of asparaginase to continue treatment in Brazil.
It should be noted that the ALL-BFM-IC protocol advocates changing the type of l-asparaginase when hypersensitivity reactions occur. Adherence to this guideline was not feasible in the hospital of this study due to the cost and limitations related to the importation and the use of other types that are not authorized by the Brazilian national health surveillance agency. When the protocol was updated in 2013, EcA was no longer indicated as the first choice for the treatment of ALL, since, of the three types, it was considered the most immunogenic.14,23
Concomitant use of corticosteroids and vincristine is considered a protective factor for hypersensitivity reactions.10 However, there was a higher incidence of reactions in patients who received vincristine together with asparaginase, which may be related to the routine of shortening the interval between the administrations of these drugs at the study site. Administration of vincristine and asparaginase within less than 12h increases the risk of neurotoxicity and reactions to vincristine. Asparaginase is a hepatotoxic drug and vincristine undergoes hepatic metabolism.24 The possible increase in reactions to vincristine may be a confounder in the diagnosis of reactions to asparaginase.
Discontinuation of treatment on suspicion of a hypersensitivity reaction to asparaginase may have been unnecessary. The analysis of the descriptions of the suspected adverse reactions suggests that, in some of the cases, hyperammonemia reactions may have occurred, whose management is possible. The increase in serum ammonia levels after the intravenous administration of asparaginase occurs in children at concentrations of 260–700μmol/L17 (reference value up to 30μmol/L). Hyperammonemia can cause nausea, vomiting, headache, restlessness, dizziness and rash,8,25 which were observed in 13 cases of this study.
Grade 3 (bronchospasm and/or angioedema) and Grade 4 (anaphylaxis) hypersensitivity reactions are easily diagnosed. Van der Sluis et al. recommend the replacement of asparaginase when severe reactions are observed. However, it seems possible to have reactions classified as Grade 2, with manifestations similar to allergies, without the formation of antibodies, as demonstrated by Kloss et al.25 According to these authors, the reactions related to antibody formation occur, in general, at the beginning of the infusion.
The identification that high risk and low age of patients are more likely to develop infusion-related reactions also indicates the need for a differential diagnosis of these reactions, since early treatment discontinuation further increases risk in these two groups.
It is known that high-risk patients are exposed to high doses of asparaginase (25,000IU/m2) in the consolidation phase of remission, which does not occur in treatments of children with other risk classifications.15 In addition, Liu et al.26 demonstrated in their work that hypersensitivity reactions are more common in less intensive regimens such as those applied to non-high-risk patients in the present study. This finding differs from the present study. An important difference between the two studies is that administration was IV in the current study whereas an IM administration was used in the other; this reinforces the possibility of hyperammonemia and non-allergic hypersensitivity reactions among those considered allergic hypersensitivity, as ammonia levels rise rapidly during asparaginase infusion by IV administration and there is the possibility of anaphylactoid reactions related to the IV route.12,24
Concerning age, children undergo intense physiological changes in the first years of life that culminate in differentiated pharmacokinetics in the different stages of childhood,27 this fact can modify the incidence of reactions related to plasma concentration of drugs and metabolites due to modifications in metabolization, excretion or distribution, but not in reactions such as hypersensitivity, that are not dose dependent. In their work, Jorck et al.24 considered hyperammonemia following the administration of asparaginase as serum levels above 80μmol/L in neonates and 100μmol/L in children older than one year, demonstrating the greater susceptibility of younger individuals to hyperammonemia reactions.
The findings of the present study suggest that early withdrawal of EcA due to suspected hypersensitivity may be mistaken because of the possibility of hyperammonemia or non-allergic hypersensitivity reactions. In this sense, the dosage of ammonia before and after asparaginase infusion and the classification as to the grade of the suspected hypersensitivity reaction are proposed strategies to monitor and in the differential diagnosis of the reactions that can guide clinical staff in the decision of continuing or interrupting treatment with EcA. Burke et al. report elevated serum ammonia levels as evidence of non-antibody-mediated reactions during intravenous asparaginase infusion.28
The dosage of ammonia is cheap (R$ 4.20–less than US$ 1.50) and the technique used is simple (colorimetric test of dry chemistry using the bromophenol blue reagent). Measurement of ammonia also permits the analysis of the inactivation of the enzyme, as ammonia serum levels can help to infer about enzyme activity.23 A marked increase in ammonia levels is expected in patients who do not show antibodies, since ammonia is one of the products of the reaction catalyzed by asparaginase and antibodies are associated with minor increases of ammonia.
The translation of the research results into the practice of the service allowed the implementation of an active pharmacovigilance strategy by monitoring both the administration of all infusions of asparaginase and ammonia levels. Reactions are classified according to the severity and the time after the infusion, which promotes greater safety in the identification of the reaction. This strategy also allows an indirect measure of asparaginase activity using ammonia levels due to the low cost and because this examination was adopted by the institution for other purposes.
Because this was a retrospective study, some of the suspected adverse reactions had no description of the signs and symptoms, which made it impossible to analyze the severity of all. In addition, the review of the literature and the risk factors found in the present study make it clear that not all reactions reported as allergic hypersensitivity in the clinical practice were actually this type of reaction. It can be implied that there were cases of real allergic hypersensitivity and others that were not, however, the precise classification of all reactions as to the type and severity is infeasible retrospectively.
ConclusionDue to the similarity between allergic hypersensitivity reactions and non-allergic hypersensitivity reactions and hyperammonemia, the differential diagnosis of the reactions related to asparaginase infusions using the measurement of ammonia and classification of the severity of reactions is crucial. The proper identification of each type of reaction will allow the proper management thereof.
Because of the predominance of Grade 1 and 2 reactions and the lack of classification of many of the reactions that occurred during the study period, it is expected that this tool will reduce the discontinuation of treatment with asparaginase.
This reduction in the number of early interruptions due to reactions that may not be related to antibody formation should positively impact the survival of individuals, since this drug is an essential component of pediatric ALL protocols.
Conflicts of interestThe authors declare no conflicts of interest.