Relapse of acute myeloid leukemia (AML) after allogeneic stem cell transplantation (allo-SCT) leads to dismal outcomes. This study aimed to identify high-risk patients and explore the effects of cytomegalovirus (CMV) reactivation in a high CMV-seropositive population.
MethodsThe study involved a single-center retrospective cohort in Thailand, analyzing clinical risk factors and CMV-mediated immune responses, correlated with transplant outcomes in AML patients.
ResultsEighty-five patients with AML in complete remission (CR) undergoing HLA-matched myeloablative allo-SCT between 2011 and February 2021 were enrolled. The relapse rate was 27.1% with the median time of 7 months after transplantation. The 3-year relapse-free-survival (RFS) and overall-survival (OS) were 72.2% and 80.8%, respectively. The disease status (>CR1) and absence of chronic graft-versus-host disease (cGVHD) were independently significant adverse prognostic factors of RFS and OS. Ninety-two percent of recipient-donor pairs were both CMV seropositive. The CMV reactivation occurred in 54.1% of the patients. The clinically significant CMV infection rate was 49.4%. No CMV syndrome/disease or CMV-related mortality occurred. One-year cumulative incidence of relapse among CMV-reactivation and non-reactivation groups were 14.3% and 25.6%, respectively, without a statistically significant difference. Transplantation-related mortality was 11.1%.
ConclusionsThe transplantation beyond CR1 and absence of cGVHD are powerful prognostic factors associated with inferior RFS and OS. In a high CMV prevalence country, there appears to be no impact of CMV reactivation on relapse in AML patients undergoing an allo-SCT.
The relapse of acute myeloid leukemia (AML) after allogeneic hematopoietic stem cell transplantation (allo-SCT) confers a poor prognosis. The prevention of leukemia relapses becomes a reasonable strategy. Prognostic factors identification is critical in selecting high-risk patients for early interventions. In previous studies, both AML-related and transplant-related risk factors have been demonstrated.1
Cytomegalovirus (CMV) reactivation after human leukocyte antigen (HLA)-matched allo-SCT has been shown to reduce AML relapse rates.2-6 The underlying immune-mediated anti-leukemic mechanisms which are enhanced by CMV reactivation have been proposed. The CMV-induced adaptive natural killer (NK) cells characterized by the NKG2C+CD57+ expression on the CD56dim+ NK cell have been reported to be associated with lower AML relapse in allo-SCT settings.7-9 In addition, a subgroup of gamma-delta T cells (Vδ2neg γδ-T cell) responding to CMV reactivation was demonstrated after allo-SCT.10 Furthermore, the T-lymphocyte, CD56+ T cell subset and NK cell reconstitutions were accelerated early after SCT by CMV viremia.8,11,12 These escalated immune cells may contribute to the protection against AML relapses.
CMV-seropositive healthy blood donors have higher numbers of adaptive NK cells (NKG2C+) and CD56+ T-lymphocytes, compared to seronegative donors.13,14 The loss of relapse prevention by pre-emptive anti-CMV therapy in a region with a high CMV prevalence was reported.15 Therefore, the CMV-influent immune activation response in a high CMV-seropositive population may be different from impacts in low CMV rate countries. This study explored clinical factors, including CMV-reactivation, which were associated with transplant outcomes and characterized CMV-mediated immune response after HLA-matched myeloablative allo-SCT for AML patients in a high CMV prevalence country.
MethodsThe retrospective cohort study enrolled AML patients who underwent HLA-matched myeloablative allo-SCT after complete remission at the King Chulalongkorn Memorial Hospital in Bangkok, Thailand from 2011 to February 2021. Thailand has a high CMV prevalence. The use of ATG-containing regimens was an exclusion criterion. The protocol was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
The transplantation protocol included a myeloablative conditioning regimen with cyclophosphamide plus total body irradiation (Cy-TBI), or busulfex plus cyclophosphamide (Bu-Cy), or fludarabine plus 4-day busulfex (Flu-Bu4). The graft-versus-host disease (GVHD) prophylaxis regimen was the calcineurin inhibitor and methotrexate, or post-transplantation cyclophosphamide plus cyclosporine and mycophenolate mofetil. The infectious prophylaxis protocol was comprised of acyclovir prophylaxis for herpes virus and fluconazole for primary fungal prevention or voriconazole for secondary aspergillosis prevention. No anti-bacterial and anti-CMV (letermovir) prophylaxis protocol was prescribed. The acute GVHD (aGVHD) grading system used the modified Glucksberg criteria and the chronic GVHD (cGVHD) assessment used the NIH 2005 criteria.
The post-transplantation CMV-viral load (VL) monitoring was scheduled weekly, following white blood cell engraftment for 2 to 3 months post-SCT. The reactivation was defined as plasma DNA of CMV-VL > 500 copies/ml, with detection at least two times. For patients taking high-dose corticosteroids, the criteria of plasma CMV-VL > 100 copies/ml was defined as positive reactivation. The preemptive therapy with ganciclovir or valganciclovir was initiated when CMV reactivation occurred. The clinically significant CMV infection was defined as CMV viremia leading to preemptive treatment, CMV syndrome or CMV disease.
The T and NK cell immunophenotypes were performed in thawed peripheral blood mononuclear cells from the available twenty-seven patients on day 90 post-SCT, in addition to five healthy volunteers. The single cell suspension was incubated with fluorochrome-conjugated monoclonal antibodies in the dark at 4°C for 30 minutes. Cells were washed 2 times and then analyzed on the BD FACSCanto II flow cytometer. The dead cells were excluded, using 7-amino-actinonycin D (7-AAD) staining. The data were analyzed on the FlowJo software. The fluorochrome-conjugated monoclonal antibodies used included: CD56-PE (clone REA196, MACS), NKG2C-VioBright FITC (clone REA205, MACS), CD57-APC (clone REA769, MACS), PD1-APC (clone EH12.2H7, Biolegend), CD3-APC-Vio770 (clone BW264/56, MACS) and TCRγδ-FITC (clone 11F2, MACS).
The clinical data were analyzed to calculate the cumulative incidence of leukemia relapse (CIR), relapse-free survival (RFS) and overall survival (OS). The RFS was defined from the date of stem cell infusion to the date of a relapsed disease or death and measured in days. The OS was defined from the date of stem cell infusion until death from any cause and measured in days. We used the Kaplan–Meier method to estimate the probabilities of RFS and OS up to 3 years. Thereafter, we explored factors associated with RFS and OS using the Cox Regression. The linearity of continuous covariates against the hazard function was assessed and, in the case of non-linearity, the covariate was modelled in quartiles; adjacent quartiles, collapsed in hazard ratio (HR) and 95%CI, were similar. Covariates with p < 0.1 were adjusted for, in a multivariate model and p-values < 0.05 were considered statistically significant. The data were analyzed using the Stata15 (Statacorp, College Station, TX, USA).
ResultsPatient characteristicsEighty-five patients with AML in complete remission undergoing HLA-matched myeloablative allo-SCT were enrolled. The median age was 41 years (range 15 – 56). The majority of AML patients (72.9%) were stratified into the intermediate risk group, using available cytogenetic and molecular studies.16 Nine patients had missing cytogenetic data and were included in the intermediate risk group. The mutations of nucleophosmin1 (NPM1) and FLT3 internal tandem duplication (FLT3-ITD) were evaluated in fourteen patients. All nine FLT3-ITD positive patients did not receive the FLT3 inhibitor because of the non-reimbursement medication. There were six 3+7 regimen-refractory patients and eight secondary AML patients. Transplantation beyond the first CR (> CR1) was performed in 13 relapsed AML patients (15.3%). Ninety-two percent of recipient-donor pairs were both CMV seropositive. All patients received peripheral blood stem cells (PBSCs) from either HLA-matched related donors (91.8%) or HLA-matched unrelated donors (8.2%). The calcineurin inhibitor plus short-course methotrexate was prescribed for GVHD prophylaxis for 78 patients (91.8%). The baseline characteristics are presented in Table 1.
Baseline characteristics of AML patients in remission who underwent allogeneic stem cell transplantation.
CMV: cytomegalovirus; GVHD: graft-versus-host disease; Cy: cyclophosphamide; TBI: total body irradiation; PT-Cy: post-transplantation cyclophosphamide; MMF: mycophenolate mofetil
The CMV reactivation occurred in 46 patients (54.1%) with no CMV-related mortality. The median reactivation time was 41 days (range 21 – 185). Forty-two patients (91.3%) received ganciclovir/valganciclovir anti-CMV therapy, with all outcomes judged as a complete response. The duration of anti-CMV treatment was 2 to 3 weeks. Most of the patients tolerated only a 2-week induction treatment, due to drug-induced cytopenia. Four untreated patients resolved the CMV reactivation after tapering down their immunosuppressive drug. No CMV syndrome or CMV disease occurred among the CMV reactivation patients. Only two cGVHD patient experienced a late CMV reactivation. Twenty-two patients were diagnosed as aGVHD (25.9%). Six of them had grades II to IV aGVHD. The cGVHD incidence was 48.2%, with 63.4% having mild cGVHD severity. Among 85 patients, eighteen patients died (21.2%). The causes of death were relapsed AML for 16 patients and infectious complications due to cGVHD treatment for 2 patients. The transplantation-related mortality (TRM) occurred in 2 deaths (11.1%).
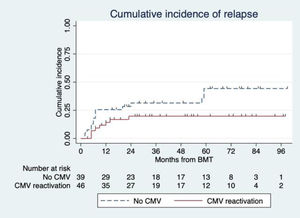
Relapsed AML occurred in twenty-three patients (27.1%), with a median time of 7 months (range 2 – 59) after transplantation. Sixteen relapsed AML patients (69.6%) were diagnosed within the first year of transplantation. One-year CIR of CMV-reactivation and non-reactivation groups were 14.3% and 25.6%, respectively. Data did not show a statistically significant association between CIR and CMV reactivation. The hazard ratio (HR) was 0.47 with a 95% confidence interval (CI) of 0.20 to 1.11 (p = 0.09) (Figure 1.).
The median follow-up time was 33 months (range 2 – 99). The 3-year RFS and 3-year OS were 72.2 and 80.8 percent, respectively. The univariate analysis of the RFS and OS demonstrated that advance disease status (> CR1), absence of cGVHD and failure of 3+7 induction were associated with an inferior outcome. The age, conditioning regimen, donor type, aGVHD and CMV reactivation were not associated with survival. By multivariate analysis, the disease status (> CR1) and absence of chronic graft-versus-host-disease were independently significant adverse prognostic factors for the RFS and OS, as shown in Table 2. For the RFS, The HR of the transplantation beyond the first CR and cGVHD were 5.13 (95% CI 2.04 – 12.87; p < 0.001) and 0.13 (95% CI 0.05 – 0.42; p < 0.001), respectively. For the OS, the HR of transplantation beyond the first CR and cGVHD were 4.10 (95% CI 1.53 – 11.04; p = 0.005) and 0.20 (95% CI 0.06 – 0.63; p = 0.006), respectively.
Univariate and multivariate Cox regression showing hazard ratios (HRs) for relapse-free survival and overall survival after transplantation in AML patients.
*HR: hazard ratio; aHR: adjusted hazard ratio; RFS: relapse-free survival; CR: complete remission; CMV: cytomegalovirus; GVHD: graft-versus-host disease; CI: confidence interval
The absolute lymphocyte count (ALC) for the 85 patients on both day 90 and day 180 post-SCT showed no significant differences between the CMV-reactivating and non-reactivating groups. The 90-day ALC and 180-day ALC of the CMV reactivation patients were 1,621.7 ± 114.0 and 2,160.9 ± 141.1 (mean ± SE, cells/μL), respectively. The 90-day ALC and 180-day ALC of non-reactivation patients were 1,707.6 ± 136.3 and 1,866.2 ± 135.2 (mean ± SE, cells/μL), respectively. Analysis of the lymphocyte subsets and the CMV-induced adaptive NK cells (NKG2C+CD57+ NK cell) on day 90 showed no significant differences between the CMV-reactivating and non-reactivating groups (Table 3).
Immunophenotypic characteristics of allo-SCT patient and healthy volunteer blood samples.
Allo-SCT: allogeneic stem cell transplantation; CMV: cytomegalovirus; SEM: standard error of mean; NK: natural killer; PD1: programmed cell death protein1
In this study, the strongest independent factor associated with an inferior RFS and OS after allo-SCT was transplantation beyond the first CR. The primary refractory to the 3+7 regimen also tended to yield a poor RFS and OS, although when controlled for >CR1 and cGVHD, the significance was lost. These findings are consistent with previous studies and support the linkage of these factors with chemotherapy resistance.1 The unfavorable cytogenetic risk was not detected as a significant factor in this cohort, possibly due to the limited number of patients. In addition to leukemic risk factors, the cGVHD was a significant prognostic factor associated with a better RFS and OS in our study, similar to many previous reports.1 This referred to the graft versus leukemia effect. The TRM was low in our cohort. The major cause of death was relapsed AML, which accounted for 88.9% of deaths.
In our center in Thailand, which has a high CMV prevalence, no difference in the CIR between the CMV-reactivation and non-reactivation was demonstrated. Due to the small number of subjects, a weak association cannot be excluded. Existing literature remains conflicting. Although a meta-analysis showed a lower relapse risk with the CMV replication,3 a large multicenter registry (n = 5,310) reported no preventive effect from the CMV reactivation in AML patients after allo-SCT.17 Heterogeneous results may depend on some variables that abrogate this advantage, for example, antithymoglobulin,18,19 reduced-intensity conditioning regimen 20 and antiviral treatment.15 The intensity of CMV-activated immune responses are possibly more important for relapse protection than the CMV itself. The early pre-emptive CMV therapy may be partly responsible for the lack of protection in our study. The low threshold for initiating the anti-CMV treatment in our cohort also led to 49.4% of patients having a clinically significant CMV infection. No CMV syndrome/disease and CMV-related mortality occurred. Although letermovir has been approved and shown efficacy and safety for CMV prevention in seropositive allo-SCT patients,21,22 the effects of this prophylactic strategy and CMV-related relapse protection requires more investigation. In Thailand, letermovir is currently not yet available.
The characterization of CMV-related immune response was performed in several blood samples. An increase in the CD56+ T lymphocyte and CMV-induced adaptive NK cells (NKG2C+CD57+) was detected without statistical significance in the CMV reactivation patients. The CD56+ T lymphocyte was previously described as the adaptive T cell subset that increased in response to the CMV replication after both an allo-SCT setting 8,23 and CMV-seropositive healthy donors.14 Consistent with our findings of NK cells, a recent study also reported no significant difference in the NK-cell reconstitution on day 100 between the CMV-reactivation and non-reactivation of CMV-seropositive patients, using the PBSC as the graft source.24 The major limitation of our study were small numbers for sample analysis and the lack of a functional analysis of the CMV cell-mediated immunity.25
The limitations of this study are the lack of minimal residual disease information and some gene mutational data. This study also conducted before the FLT3 inhibitor was combined with the treatment regimen at our center. In addition, the high probability of the survival outcome in this cohort may be influenced from some biases of baseline characteristics, such as young age, low proportion of poor-risk patients and use of myeloablative regimen.
ConclusionIn summary, our study found that transplantation beyond the CR1 and absence of cGVHD are powerful prognostic factors associated with an inferior RFS and OS. With a high CMV prevalence in the Thailand setting, no impact of the CMV reactivation on the relapse in AML patients undergoing an allo-SCT was found in our study.
We would like to express our gratitude to Dr. Stephen J Kerr, who helped us analyze the statistics. We would like to acknowledge Dr. Koramit Suppipat for supporting us with some fluorochrome-conjugated antibodies.