Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML) marked by a high incidence of coagulopathy. Podoplanin, a glycoprotein involved in platelet activation through interaction with CLEC-2, has recently been identified on leukemic promyelocytes and suggested as a potential contributor to APL coagulopathy. Identification of novel biomarkers and therapeutic targets for APL coagulopathy can potentially improve the outcomes of this condition
AimTo explore whether levels of soluble podoplanin in plasma are different in APL, and to evaluate its association with laboratory and clinical outcomes in these patients
MethodsSamples were obtained from consecutive patients with APL at the time of diagnosis in an academic hospital. Biobank samples from 35 patients with non-APL AML matched for age and sex were used as comparators. Circulating podoplanin levels were measured in plasma using a commercial ELISA kit. The study was approved by the institutional ethics committee and all participants provided written informed consent
ResultsAPL patients showed significantly higher plasma soluble podoplanin concentrations compared to non-APL AML. Using the median soluble podoplanin value as a cutoff, a higher proportion of APL patients presented elevated levels. Soluble podoplanin levels correlated with CD40L in APL cases, but not in non-APL AML patients, suggesting a possible interaction with thrombo-inflammatory activation pathways
ConclusionThese findings represent a proof-of-concept that measuring soluble podoplanin in plasma samples can contribute to the diagnosis of APL, while also providing novel data on the association of podoplanin with the pathogenesis of APL coagulopathy.
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia (AML) characterized by the accumulation of promyelocytes in the bone marrow and a high risk of severe hemorrhagic complications. Despite significant therapeutic advancements with all-trans retinoic acid (ATRA) and arsenic trioxide (Arsenic Trioxide) [1–3], early mortality remains a major concern, potentially reaching 5–20 %, depending on clinical and geographical factors. The primary cause of early death is coagulopathy, which often leads to fatal intracranial hemorrhages [4]. Therefore, early diagnosis and prompt initiation of treatment are crucial for reducing mortality and improving patient outcomes. In addition, the identification of novel biomarkers and therapeutic targets of APL coagulopathy could bring benefits to these patients.
Podoplanin is a small transmembrane glycoprotein expressed in various cell types, including lymphatic endothelial cells, kidney podocytes, and certain tumor cells [5]. Functionally, podoplanin interacts with the C-type lectin-like receptor 2 (CLEC-2) on platelets, triggering their activation and contributing to thrombotic processes [6]. This mechanism is particularly relevant in cancer-associated thrombosis and inflammatory diseases, where podoplanin overexpression has been linked to prothrombotic states [5–7].
In the context of APL, podoplanin expression was first identified in leukemic promyelocytes in 2018, suggesting its potential role as a distinguishing marker for this leukemia subtype [8]. More recently, its expression was validated in a prospective cohort using flow cytometry, reinforcing its diagnostic and pathophysiological relevance in APL [9]. However, the extent to which circulating podoplanin correlates with disease characteristics and coagulation abnormalities remains unexplored.
Soluble forms of membrane proteins can serve as biomarkers, reflecting their biological activity and disease status. For instance, soluble fms-like tyrosine kinase-1 (sFLT1) is well established as a biomarker in preeclampsia and soluble urokinase-type plasminogen activator receptor (suPAR) as a biomarker in different types of cancers [10–13]. Similarly, soluble podoplanin (sPDPN) has been identified as a biomarker in malignancies and inflammatory diseases such as cancer and COVID-19, highlighting its potential and prognostic significance [14–18].
Based on this evidence, this study investigated whether quantification of sPDPN in plasma could be associated with the diagnosis of APL, as well as its relationship with biomarkers involved in APL coagulopathy.
MethodsStudy populationThis study analyzed patients diagnosed with acute leukemia between 2014 and 2022 at an academic tertiary hospital. Inclusion criteria were: age ≥18 years and a confirmed diagnosis of APL or AML. Exclusion criteria included: presence of severe infection with hemodynamic instability requiring vasoactive agents at the time of sample collection, and a diagnosis of other forms of acute leukemia. Patients with non-APL AML were used as a comparator group. These patients were selected from the population of consecutive new AML cases during the same study period, matched for age, sex and year of enrollment, before any of the laboratory analyses. The diagnostic workup comprised morphological evaluation, immunophenotyping, cytogenetic analysis, and molecular testing. APL cases were confirmed through molecular detection of the PML-RARA fusion gene. Patients with a strong clinical and morphological suspicion of APL, based on peripheral blood smear findings, were promptly initiated on ATRA therapy in accordance with the institutional protocol. This study was approved by the institutional ethics committee (CAAE: 39,948,520.8.1001.5404) and performed in accordance with the Declaration of Helsinki.
Sample collection and processingWhole blood samples were collected in EDTA tubes. Plasma was obtained by centrifugation at 2500 g for 15 min at 22 °C within two hours of collection. All samples were aliquoted and stored at −80 °C until analysis.
Clinical and laboratory dataClinical and laboratory data were retrospectively retrieved from hospital electronic medical records. Laboratory variables included initial bone marrow blast percentage, hemoglobin concentration, white blood cell (WBC) count, platelet count, prothrombin time (TP), activated partial thromboplastin time (aPTT), and fibrinogen concentration, based on the first set of results available upon hospital admission. Clinical outcomes comprised early mortality (defined as death within 30 days of diagnosis) [19] and central nervous system (CNS) and retinal bleeding.
Measurement of soluble podoplanin levelsPlasma levels of podoplanin were measured in duplicate in EDTA plasma using a commercial enzyme-linked immunosorbent assay kit (ELISA) (Sigma Aldrich, Cat. RAB 1632–1KT - Lot: 0325/2117), according to the manufacturer’s instructions.
Measurement of P-selectin and CD40LPlasma concentrations of P-selectin and CD40L were determined using a custom-designed multiplex assay panel (Invitrogen – Thermo Fisher Scientific – Cat. PPX-07-MX323GU – Lot: 283,814–000), following the manufacturer’s protocol. All measurements were performed in duplicate, and analyte concentrations were calculated based on standard curves generated using known concentrations of recombinant proteins.
Statistical analysisQuantitative variables were described using both median with interquartile range and mean with standard deviation (SD). Patients with APL were matched 1:1 to non-APL AML patients, as detailed above. Matching was done prior to any laboratory analysis. Comparative analyses between groups were performed using parametric and nonparametric statistical methods. The Student’s t-test was applied for normally distributed data, while the Mann-Whitney U test was used for data not meeting parametric assumptions. For the analysis of the association between podoplanin expression with clinical outcomes, APL patients were arbitrarily categorized as podoplanin positive or negative, based on the 50th percentile cutoff. Correlations were evaluated by Spearman's test. A p-value <0.05 was considered statistically significant. Statistical analyses were conducted using IBM SPSS Statistics version 26 and GraphPad Prism version 8.0.
ResultsIn total, 35 patients with APL were included in this study, as well as 35 patients with non-APL AML, matched by age, sex and year of diagnosis, used as a comparator group. The clinical and laboratory characteristics of these groups are presented in Table 1. As expected, APL patients presented more frequently with alterations in hemostasis biomarkers, and a lower rate of early mortality.
Laboratory and clinical characteristics of study participants.
| APL(n = 35) | Non-APL AML(n = 35) | p-value‡ | |
|---|---|---|---|
| Age* | 37.3 (21–61) | 41.4 (18–59) | 0.31 |
| Male:Female | 17:18 | 17:18 | 0.99 |
| Risk group - (low/int/high) | 3/18/14 | 11/11/13 | 0.04 |
| Peripheral blast count, 109/L* | 2.23 (0.01–129.6) | 7.75 (0.1–130.8) | 0.25 |
| Bone marrow blast count - %* | 88.0 (46.0–98.2) | 80.5 (26.5–95.6) | 0.002 |
| Leukocytes - 103/µL* | 3.1 (0.32–137.9) | 14.4 (0.91–150.3) | 0.02 |
| Hemoglobin - g/dL⁎⁎ | 8.4 ± 2.39 | 8.1 ± 2.10 | 0.64 |
| Platelets - 109/L* | 22.0 (4.0–89.0) | 27.0 (5.0–230.0) | 0.02 |
| PT (INR)⁎⁎ | 1.29 ± 0.19 | 1.19 ± 0.13 | 0.01 |
| aPTT* | 0.90 (0.75–1.24) | 0.89 (0.67–1.38) | 0.48 |
| Fibrinogen - mg/dL* | 121.7 (26.0–378.5) | 384.5 (58.9–705.8) | <0.0001 |
| Clinical outcomes | |||
| CNS/retinal bleeding - n ( %) | 4 (11.4) | 2 (5.7) | 0.39 |
| 30-day survival - (no/yes) | 5/30 | 12/23 | 0.05 |
Mann-Whitney test, t-test or chi-square test.
APL: acute promyelocytic leukemia; AML: acute myeloid leukemia; CNS: central nervous system; PT: prothrombin time; aPTT: activated partial thromboplastin time; INR: International Normalized Ratio
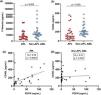
Plasma concentrations of sPDPN ranged from 0.3–113.6 ng/mL. Patients with APL exhibited significantly higher levels of sPDPN (median: 16.4 ng/mL) compared to patients with other AML subtypes (median: 2.2 ng/mL; p-value = 0.003) (Figure 1).
Association of podoplanin expression with clinical outcomes in acute promyelocytic leukemia.
| Clinical outcome | Podoplanin expression category* | p-value⁎⁎ | |
|---|---|---|---|
| Podoplanin + | Podoplanin - | ||
| Early mortality (30-day) | 0.46 | ||
| Yes | 4 | 1 | |
| No | 19 | 11 | |
| CNS/ retinal bleeding | 0.67 | ||
| Yes | 3 | 1 | |
| No | 20 | 11 | |
CNS: Central nervous system.
APL coagulopathy still represents a major challenge to the early management of APL, and the availability of biomarkers capable of differentiating APL from other forms of AML, and of providing information about APL coagulopathy could potentially facilitate the management of these patients [20]. The main contribution of the present study was the demonstration of sPDPN as a potential biomarker. Its expression is markedly higher in APL compared to other forms of AML, and it correlates with an important thrombo-inflammatory mediator. This suggests that podoplanin expression may be a unique biological feature contributing to the well-known predisposition of these patients to hemorrhagic and thrombotic complications.
The first demonstration that podoplanin expression as a hallmark of APL was published in 2018 and included data obtained by flow cytometry and RNA sequencing [8]. That study also presented evidence linking podoplanin expression to platelet activation in vitro. While the diagnosis of APL using flow cytometry and molecular techniques is well established, reliance on methods that are not usually available outside specialized cancer centers may delay diagnosis and jeopardize patient outcomes [20]. Immune-based methods such as ELISA are widely available and have the advantage of being potentially performed with diagnostic kits in almost every setting. Based on the demonstration that circulating levels of podoplanin was capable of identifying discrete subgroups of patients with other conditions [14–18], and that soluble levels of membrane proteins can serve as useful disease biomarkers [10–13], we hypothesized that sPDPN levels might contribute to the differential diagnosis of APL from other forms of AML. In fact, this study demonstrates a significant difference in sPDPN levels between these two patient subgroups, although a subgroup of patients with non-APL AML also presents higher sPDPN values.
In addition, a positive correlation between sPDPN and CD40L levels was found in APL patients, suggesting a biological interaction between these two markers. CD40L is a transmembrane protein expressed on activated platelets that has been implicated in platelet-leukocyte crosstalk and inflammatory signaling [21]. CD40L is also expressed by other hematopoietic cells such as lymphocytes, neutrophils, dendritic cells, monocytes and macrophages, in response to cytokines [22], which are key elements of thrombo-inflammatory pathways [23]. So, the correlation described in the current study could represent either evidence of the participation of podoplanin in platelet activation, in alignment with Lavallé et al. [8], who demonstrated that podoplanin-expressing APL blasts can activate platelets, or a yet unknown association between podoplanin and CD40L in another hematopoietic compartment. In the present dataset, sPDPN levels were not associated with clinical outcomes, although this study was not powered for this type of analysis.
The results also demonstrate that a significant proportion of AML patients present detectable sPDPN levels, while some APL patients present low sPDPN levels. This is in contrast with data from flow cytometry and reverse transcription polymerase chain reaction (RT-PCR) results from our lab that show less overlap between APL and other forms of AML (data not shown). Additional studies are warranted to explore and explain this lower accuracy of sPDPN ELISA for the segregation of APL from other forms of AML compared to flow cytometry.
This study has limitations that should be acknowledged. Although APL is a relatively rare condition and the present sample size was sufficient to demonstrate relevant biological differences in sPDPN levels, this study was not powered to analyze clinical outcomes, which were rare. Another limitation lies in the absence of additional hemostatic biomarkers, which might have enhanced the interpretation of coagulation-related mechanisms. However, these markers are not routinely assessed in the standard clinical workflow of the hospital, and citrate plasma samples were no longer available. Future studies involving larger cohorts and broader biomarker panels are important to further investigate the potential diagnostic and clinical relevance of sPDPN in APL.
In conclusion, the results of this study represent proof-of-concept that the immunodetection (in this case, using ELISA) of circulating levels of sPDPN can serve as a complementary tool in the differential diagnosis of APL. The data also adds support to the concept that podoplanin expression is a distinct hallmark of APL and contributes to the pathogenesis of the unique thrombo-inflammatory complications seen in these patients.
Plasma levels of podoplanin of acute promyelocytic leukemia (APL) and non-APL acute myeloid leukemia (AML) patients (p-value: Mann-Whitney test) To investigate the potential relationship between sPDPN and platelet activation, plasma levels of P-selectin and CD40L, two established markers of platelet degranulation, were measured. Both P-selectin (7194 ng/mL versus 11,220 ng/mL; p-value = 0.003) and CD40L levels (100.4 ng/mL versus 196.6 ng/mL; p-value = 0.002) were both significantly lower in APL patients compared to those with other AML subtypes (Figure 2A, B). Interestingly, a significant positive correlation was observed between podoplanin and CD40L levels in APL patients (Rs = 0.60; p-value = 0.0004), but not for other AML subtypes (Figure 2C,D). Podoplanin levels were not associated with predefined clinical outcomes (Table 2).
(A) Plasma levels of P-selectin were significantly lower in acute promyelocytic leukemia (APL) patients compared to non-APL acute myeloid leukemia (AML) patients (Mann-Whitney test; p-value = 0.003). (B) Plasma levels of CD40L were significantly lower in APL patients compared to non-APL AML patients (Mann-Whitney test; p-value = 0.002). (C) A significant positive correlation between podoplanin and CD40L was observed in APL patients (Spearman’s correlation; p-value = 0.0004). (D) No significant correlation between podoplanin and CD40L was found in non-APL AML patients.
All reported data are available for sharing upon a reasonable request to the corresponding author.
Ethics statementThe study was performed in accordance with the Declaration of Helsinki and approved by the local institutional research board (protocol CAAE: 39,948,520.8.1001.5404).
Author contributions statementCRPM: performed all assays; revised medical records; contributed to data analysis and drafted the manuscript; revised and approved the manuscript. CMAS: revised medical records; contributed to data analysis; revised and approved the manuscript. ITBJ: contributed to data analysis; revised and approved the manuscript. BKLD: contribute to study design; revised and approved the manuscript. PMC: provided laboratory support and infra-structure; revised and approved the manuscript. STOS: provided laboratory support and infra-structure; revised and approved the manuscript. EVDP: designed the study, oversaw and provided resources and infra-structure for ELISA analysis, contributed to data analysis; drafted the manuscript; revised and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.