Adult T-cell leukemia/lymphoma (ATLL) was first described in 1977 in Japan and is a subtype of aggressive mature T-cell neoplasm which consists of mature CD4 + CD25+ T-lymphocytes.1 The World Health Organization (WHO) 2016 classification system categorises ATLL as a peripheral T-cell lymphoma (PTCL). ATLL is caused by the human T-cell lymphotropic virus type 1 (HTLV-1) which is a novel RNA retrovirus.2 The HTLV-1 is contracted through 4 main means: vertical transmission during child delivery and breastfeeding, sexual intercourse, needle sharing and blood product transfusion.3 ATLL demonstrates a long latency period, in which the disease may occur up to 20–30 years after the onset of HTLV-1 infection. ATLL has the highest incidence in Japan, Caribbean basin and Central Africa.4 There has been no published data on the prevalence of the disease in Malaysia except for rare individual case reports. ATLL is reported to occur in individuals of 40–50 years of life.4 It is more commonly seen in males with a male to female ratio of 1.5:1. ATLL frequently carries a dismal prognosis due to chemotherapy resistance and severe functional immunosuppression. The Japanese Shimoyama classification categorizes ATLL into 4 different subtypes: acute, lymphomatous, smoldering and chronic forms.5 The acute and lymphomatous subtypes carry an aggressive course whereas the smoldering and chronic subtypes show a better prognosis. Epstein-Barr virus co-infection may be observed in the clinical course of ATLL giving rise to features such as massive lymphadenopathy, hepatosplenomegaly, skin, bone, gastrointestinal and lung infiltration, and severe hypercalcemia. Here, we report an aggressive presentation of ATLL in a middle-aged Malaysian Chinese male who demonstrated co-infection with Epstein-Barr (EBV) and Human T-lymphotropic virus-1 (HTLV-1) who subsequently succumbed to recurrent infections.
Case presentationA 59-year-old Malaysian Chinese gentleman who had no known prior medical illness presented to the department of hematology with prolonged fever, anorexia, loss of weight and night sweats for the past 2 months. He also complained of skin rash which was red and itchy. He worked as a hawker. He was a non-smoker, a teetotaler and did not consume recreational drugs. He had no significant family history. He was married with 2 children. He did not have any known drug or food allergy. He had no history of travel or blood transfusion in the past.
On examination, he was alert with a blood pressure of 130/80 and a pulse rate of 96 beats/minute. He was febrile at 39°C. There was a generalised skin rash which was erythematous, pruritic, macular and purpuric in nature affecting the face, trunk, body and all four limbs. He did not have any palpable cervical, axillary, or inguinal lymphadenopathy. His spleen was palpable at 5cm below the left costal margin. There were no other palpable organomegaly. Other organ system examinations were unremarkable.
The complete blood count (CBC) which was analysed by an automated haematologic analyser (Sysmex, XE-5000, Vienna, Austria) revealed normochromic-normocytic anemia, leucocytosis with lymphocytosis and thrombocytopenia. Serological analysis showed detection of anti-HTLV-1 antibodies. Other blood parameters are tabulated in Table 1.
Tabulation of laboratory parameters.
| Laboratory parameters | Unit (normal range in S.I. unit) |
| Haemoglobin | 7.6 (13.5−16.5g/dL) |
| Total White Cell Count | 176 (4−10×109/L) |
| Absolute lymphocyte count | 103 (1.5−4.0×109/L) |
| Platelet | 102 (150−400×109/L) |
| Lactate Dehydrogenase (LDH) | 952 (140−280 U/L) |
| Creatinine | 68 (60−90 umol/L) |
| Albumin | 38 (40−55g//L) |
| Alanine Transaminase | 34 (0−40 U/L) |
| Calcium (corrected) | 3.7 (2.2−2.6mmol/L) |
| Anti-HTLV-1 Ab | Detected |
| Anti-HIV-1.2 | Not detected |
| HepBsAg | Not detected |
| Anti-Hep C | Not detected |
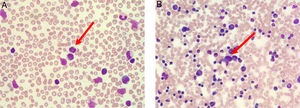
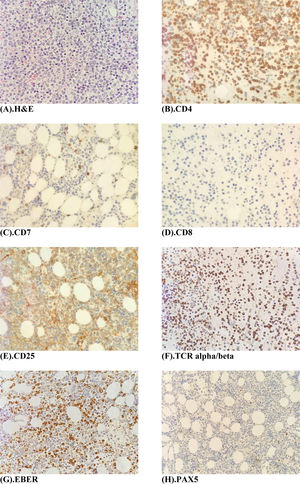
The peripheral blood film (Fig. 1A) stained with Wright-Giemsa demonstrated multilobulated lymphocytes resembling flower and clover-leaf appearance. The bone marrow aspiration (Fig. 1B) stained with May-Grunwald-Giemsa stain was hypercellular for age and showed numerous abnormal lymphocytes. The bone marrow trephine biopsy (Fig. 2A) revealed a hypercellular marrow with diffuse marrow infiltration by large clusters of abnormal lymphocytes. The neoplastic cells on the trephine biopsy (Fig. 2B–H) stained positive for CD4, CD25, TCR alpha/beta and Epstein-Barr virus encoded RNA (EBER). They stained negative for CD7, CD8 and PAX5. The immunohistochemical stain kit used was from Dako Artisan Link, Denmark. Immunophenotyping of the marrow sample by 8-colour multiparameter flow cytometer (BD FACSCanto II) showed a clonal population of 62.1% cluster of abnormal lymphocytes expressing CD2, CD3, CD4, CD5, CD25, TCR alpha/beta. The abnormal cells did not express CD7, CD8, CD26 and CD34. Cytogenetic analysis was performed utilizing the Giemsa banding technique in which 20 metaphases were analysed. They revealed extra copy of chromosome X and 3, structural abnormalities involving chromosome Xp, 4q, 6q, balanced t(11;14) and an interstitial deletion of chromosome 6q which fulfils the International Society for Cytogenetics, (ISCN) 2016 criteria for complexity. A whole-body CT imaging did not reveal any significant lymphadenopathy.
(A) Peripheral blood film (stained with Wright-Giemsa) demonstrates lymphocytosis with some lymphocytes exhibiting multilobulated appearance resembling flower-like appearance (red arrow). (B) Bone marrow aspiration (stained with May-Grunwald-Giemsa) is hypercellular for age and shows lymphocytosis with some lymphocytes exhibiting abnormal morphology (red arrow).
(A) Bone marrow trephine biopsy H & E stain (×40 magnification) shows a hypercellular marrow with diffuse infiltration by large clusters of abnormal lymphocytes. (B–F) Immunohistochemistry panel, Dako, Denmark (×40 magnifications) shows tumour cells staining positive for CD4, CD25 and TCR- alpha/beta and EBER. Tumour cells stain negative for CD7, CD8 and PAX5.
He was diagnosed with EBV/HTLV-1 co-infection acute adult T-cell leukemia (ATL-leukemic type). He was treated with oral zidovudine 300mg thrice daily and subcutaneous interferon alpha-2A 5 MU daily. However, during the course of treatment, he presented to the hospital frequently for recurrent pneumonia, in which, he was treated with intravenous antibiotics. He achieved partial remission (PR) at 2 months of therapy. At the third month of therapy, he developed progressive worsening lymphocytosis which clearly demonstrated aggressiveness of the disease and refractoriness to therapy. He became more cachexic and subsequently succumbed to severe pneumonia at 3 months of therapy.
DiscussionOur case illustrates a middle-aged Malaysian Chinese male who presented with an aggressive course of EBV/HTLV-1 co-infection acute ATL and subsequently succumbed to severe recurrent infections. He had documented presence of HTLV-1 infection which was demonstrated on his serology assessment. Besides that, the EBV-encoded RNA (EBER) in-situ hybridization (ISH) of the trephine biopsy was positive. Two-to-five percent of patients infected with HTLV-1 may develop ATLL in their lifetime.6 HTLV-1 serology is always positive in ATLL although some seronegative cases have been described. Clonal integration of the HTLV-1 provirus in neoplastic cells which is demonstrated on Southern blot analysis or inverse polymerase chain reaction (PCR) is an imperative feature of ATLL.7
The exact mechanism in which HTLV-1 induces malignant transformation and leukemogenesis is not fully understood. The expression of Tax protein leads to oligoclonal expansions of HTLV-1 infected T-cells which, in turn, activates various cellular pathways including down- signalling of nuclear factor-kappa beta (NF-kB) pathway, upregulation of anti-apoptotic proteins, activation of cAMP response binding protein and repression of p53.8 Disruption to several cell-cycle regulators including cyclins also occur as a result of the changes in the Tax protein. Tax also affects the tumour microenvironment by activating angiogenesis which facilitate the invasion of ATLL cells. The expression of HTLV-1 bZIP factor gene (HBZ) which is an antisense mRNA transcribed from the 3’ LTR is consistently present in ATL.9 HBZ may have an important role in ATLL leukemogenesis. Besides that, a proportion of patients with ATLL demonstrated Epstein-Barr virus (EBV) oncoprotein expression in neoplastic cells. It is interesting to note that EBV and HTLV-1 may infect the same T-cells through interleukin-4 (IL-4) signalling, thus, contributing to the oncogenesis of ATLL.10 IL-4 upregulates the expression of adhesion molecules via autocrine or paracrine mechanisms resulting in widespread organ infiltration and resistance to chemotherapy.10 The mechanisms involved in EBV/HTLV-1 co-infection may explain the aggressiveness of the disease in our patient.
The diagnosis of ATLL is based on the clinical presentation, morphology and flow cytometric expression of neoplastic cells, presence of HTLV-1 seropositivity and/or a histologically proven peripheral T-cell malignancy. Pronounced eosinophilia is more commonly seen in ATLL than other T-cell lymphomas. The development of eosinophilia is thought to arise from the stimulation of lymphokines such as interleukin 3 (IL-3), IL-5, transforming growth factor-beta (TGF-B) and granulocyte-macrophage colony stimulating factor (GM-CSF) by ATLL lymphocytes. On cytomorphology, tumour cells in ATLL display multilobation, also termed as flower cells or clover leaf cells which are characteristic and pathognomonic for ATLL. These cells express CD45RO, CD2, CD3, CD4, CD5, CD25 and T-cell receptor (TCR-alpha/beta) on flow cytometry.11 They are negative for CD7, CD8, CD26 and terminal deoxynucleotidyl transferase (nTDT). Our patient demonstrated complexity on conventional karyotyping which is consistent with most of the literature findings. The common cytogenetic abnormalities detected are trisomy 3, trisomy 7 and loss of chromosome X.12
Patients with ATLL are functionally immunocompromised. They frequently develop a range of opportunistic infections (OI) such as disseminated fungemia, Pneumocystis carinii pneumonia, strongyloidiasis, toxoplasmosis, neurocysticercosis, toxocariasis and cryptococcosis.13
The treatment for ATLL depends on the subtype. Acute and lymphomatous subtypes tend to be aggressive while the smoldering and chronic forms confer better prognosis. Watchful waiting or antiviral therapy is usually reserved for indolent ATLL whereas chemotherapy is given for aggressive-type ATLL. Literature remains largely scarce on specific treatment modalities for EBV/HTLV-1 co-infection ATLL as most existing robust research concentrate on HTLV-1 associated ATLL.
Antiviral therapy is the mainstay of treatment for leukemic-type ATL.14 Combined interferon-alpha and zidovudine is effective in ATLL. Patients with acute, smoldering and chronic forms of ATLL had better outcomes with antiviral therapy alone whereas chemotherapy was more efficacious in lymphoma type ATLL.15 Overall survival was 100% in patients with chronic and smoldering types of ATLL treated with antiviral therapy.15
First generation polychemotherapy such as CHOP and EPOCH have been used in aggressive ATLL. With CHOP polychemotherapy, only 16–36% achieved CR.16 In Japan, mLSG15which refers to sequential VCAP-AMP-VECP regimen consisting of vincristine, cyclophosphamide, doxorubicin, prednisolone (VCAP), doxorubicin, ranimustine, prednisolone (AMP), vindesine, etoposide, carboplatin, prednisolone (VECP) has been recommended as first line therapy in aggressive-type ATLL. The complete remission was 40% with mLSG15 as compared to only 25% in CHOP-14.16 Besides chemotherapy, allogenic stem cell transplantation (Allo-SCT) is a curative treatment modality in aggressive-type ATLL. High dose therapy-autologous stem cell transplantation (HDT-ASCT) has not shown much success in ATLL.17
Novel agents such as anti-CCR monoclonal antibody, mogamulizumab is promising in relapsed or refractory ATLL. Mogamulizumab enhances antibody-dependent cellular cytotoxicity via binding to effector cells. CCR4 expression in ATLL is associated with a poorer prognosis. Mogamulizumab is dosed at 1mg/kg as a single agent intravenously once a week for eight weeks. The overall response rates (ORR) was 50%.18 The response to mogamulizumab is usually not durable, thus requiring allogenic stem cell transplantation for curative intent.
ConclusionIn conclusion, we report a patient with EBV/HTLV-1 co-infection acute ATL (leukemic-type ATL) who presented with recurrent infections. His poor prognostic factors were male gender, leukocytosis, predisposition to recurrent infection, elevated LDH, hypercalcemia and presence of EBV co-infection. Co-infection of EBV and HTLV-1 in ATLL is an important prognostic marker and more prospective randomised controlled studies in this area may lead to better future therapeutic modalities.
Author contributionsG.K. analysed the data, designed the paper and contributed to the writing of the manuscript. J.S. made critical revisions and approved the final manuscript.
Conflicts of interestThe authors declare no conflicts of interest.