Hemorrhagic cystitis (HC) is a common complication of haploidentical hematopoietic stem cell transplantation (haplo-HSCT), characterized by irritative symptoms of the urinary tract and a higher morbidity and mortality rate. The worldwide incidence is reported between 10% and 70%. The use of alkylating agents and BK viral infection are the most frequent etiologies. The aim of this study was to report the HC incidence in an outpatient haplo-HCST program with a reduced intensity-conditioning (RIC) regimen, cataloguing risk factors, complications and final outcomes.
MethodsThe medical database of patients who received a haplo-HSCT between January 2012 and November 2017 was retrospectively analyzed. Demographic variables, general characteristics and HC incidence were included.
ResultsOne hundred and eleven patients were included, 30 (27%) of whom developed HC, most of them (70%) being grade II, with a 30-day (7–149) median time of post-transplant HC onset. The BK virus was detected in 71% of the urine samples analyzed. All HC patients responded to treatment, except two (6.6%), who died due to HC complications.
ConclusionsThere was no difference in the HC incidence or severity, compared to that reported when performing haplo-HSCT in hospitalized patients, although the donor-recipient sex mismatch did relate to a higher HC incidence.
Increasingly, haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has been used to treat malignant hematologic diseases. This has been primarily due to recent improvements in conditioning regimens and clinical outcomes, thus overcoming the lack of compatible human leukocyte antigen (HLA) donors.1,2 Nevertheless, haplo-HSCT is not free of complications; hemorrhagic cystitis (HC) is one of the most relevant, with a reported incidence of 10%–70%.3–6 This complication is a common cause of hospitalization, raising post-transplant morbidity and also mortality in these patients.
HC is an inflammatory process of the bladder, with clinical manifestations that vary from mild to severe. Clinically, the condition is characterized by hematuria, dysuria, irritative symptoms of the urinary tract and even renal failure due to hydronephrosis. HC is classified into four grades (I–IV) according to severity.7,8
In patients having received a haplo-HSCT, different risk factors and etiological agents are associated with HC, including alkylating agents, such as cyclophosphamide or busulfan, radiotherapy and infectious agents, such as the Polyomavirus BK.9–13 Cyclophosphamide is associated with early onset HC, usually 24–72 h post-drug administration.14,15
The BK is a DNA virus of the polyomaviridae family, which remains latent in urothelial cells. Primary infections occur during childhood, at a median age of 4–5 years. The worldwide BK seroprevalence in asymptomatic adults is 46%–94%.16 Nevertheless, in immunosuppressed patients, higher virus replication rates are recorded leading to cystitis, which is frequently of late onset, unlike the early-onset caused by alkylating agents.7 Additionally, other infection risk factors have been reported, including myeloablative conditioning regimens, second stem cell transplants and graft vs. host disease (GVHD).3,17
The post-haplo-HSCT HC treatment has not yet been standardized. However, common therapeutic strategies include general measures, such as oral or intravenous hydration, pain relief, cidofovir and quinolones, such as ciprofloxacin. This latter agent has been known to halt BK virus replication. Some patients, without complete response to antimicrobial therapy, benefit from steroids.18–23 In patients with severe or refractory HC, other therapeutic options include the endoscopic application of fibrin glue to damaged bladder mucosa, hyperbaric oxygen therapy, electrocoagulation of bladder mucosa, selective arterial embolization and cystectomy. However, the latter approach is associated with high complication rates and mortality and therefore, should remain as a last resource.24–29 In the literature, information on HC after haplo-HSCT is scarce, in terms of treatment options and clinical outcomes, and this is especially true for the outpatient, reduced-intensity conditioning setting.
We described HC incidences in a group of patients who received a haplo-HSCT on an outpatient basis. We assessed HC risk factors, such as donor and recipient gender, CD34+ doses, GVHD, treatment and outcomes.
Materials and methodsPatient informationA retrospective analysis was made of the medical database of patients who had received a haplo-HSCT in the Hematology Department of the Dr. José E. González University Hospital and School of Medicine of the Universidad Autónoma de Nuevo León in Monterrey, Mexico between January 2012 and November 2017. Due to the retrospective nature of the study, it was not necessary to request informed consent from the patients, but patient confidentiality was strictly maintained. All patients and stem cell donors signed an informed consent before beginning the transplant process, according to the institutional and FACT guidelines. Patients were divided into two groups, according to the presence or absence of HC. Demographic variables and general characteristics of both groups were obtained and analyzed. HC severity was classified according to the following criteria: grade I, microscopic hematuria; grade II, macroscopic hematuria; grade III, hematuria with clots, and; grade IV, hematuria with blood clots, urinary tract obstruction, clot removal or renal failure due to obstructive nephropathy.3,4 Additionally, the HC onset was classified as early or late if the condition started before or after 72 h into the post-conditioning regimen.30 The quantitative polymerase chain reaction (qPCR) method was used to detect the BK virus in urine or blood samples.
Outpatient transplant procedureHematopoietic stem cell mobilization and collectionPeripheral blood was the source of hematopoietic stem cells, with apheresis performed on an outpatient basis in all cases. The mobilization of hematopoietic stem cells in all HLA-haploidentical donors was performed using subcutaneous filgrastim at 10 µg/kg/day for four consecutive days. Stem cell collection was performed by one apheresis procedure on the fifth day of mobilization, with a continuous flow system, by means of an Amicus® separator system (Fresenius-Kabi, Germany). The objective of apheresis was to process five donor blood volumes to generate ≥2 × 106/kg CD34+ hematopoietic stem cells. Hematopoietic stem cells obtained were measured by flow cytometry in a Beckton Dickinson Accuri C6 flow cytometer (BD Biosciences, USA), using a BD stem cell enumeration kit, according to the International Society of Hemotherapy and Graft Engineering (ISHAGE) guidelines. In all cases, the cells were collected and transplanted the same day (day "0"), without cryopreservation.
Conditioning regimen and GVHD prophylaxisA conditioning regimen was chosen according to patient disease status. Patients with malignant hematologic disease received a conditioning regimen based on cyclophosphamide (350 mg/m2/day) and fludarabine (25 mg/m2/day) on days −5 to −3 and melphalan (100 mg/m2/day) on days −2 and −1. Patients with non-malignant disease received cyclophosphamide (350 mg/m2/day) and fludarabine (40 mg/m2/day) on days −5 to −3, with a low dose of melphalan (70 mg/m2/day) on days −2 and −1. The prophylactic scheme for GVHD consisted of cyclophosphamide at 50 mg/kg/day on days +3 and +4 post-transplantation, in addition to oral cyclosporine (6 mg/kg/day) and mycophenolate (1 g daily).
The prevention of uroepithelial damage by cyclophosphamide was mediated by Mesna (80% of the cyclophosphamide dose) and hydration (1.5 L/m2), from commencement to six hours after the end of the cyclophosphamide infusion. Hydration (50%) was administered intravenously and the other 50% orally at home, as all patients were followed on an outpatient basis. For infection prophylaxis, oral levofloxacin at 500 mg/day, acyclovir at 400 mg/day and itraconazole at 100 mg/day were administered to all patients from day zero, until >500 granulocytes/µl of peripheral blood were present. Then levofloxacin was changed to trimethoprim-sulfamethoxazole to prevent the Pneumocystis carinii infection. All patients remained in the outpatient treatment area during the medication infusion and were subsequently released to their homes, with daily follow-up until full hematological recovery was achieved. In sequence, they underwent a weekly follow-up.
Statistical analysisPatient characteristics were summarized using tendency measures. The Kolmogorov–Smirnov test was used to determine data distribution. The Mann–Whitney U test was performed to correlate numeric nonparametric variables and the Chi–squared test was used for categorical variables. All analyses were performed using the SPSS IBM Statistics version 22.0 software.
ResultsOne hundred and eleven patients were included, 72 males and 39 females, with a median age of 18 (1–63 years). A total of 85% of the patients had a malignant hematologic disease, the most frequent diagnosis being acute leukemia (71%). All patients received a haplo-HSCT because they did not have fully compatible HLA donors. General patient and clinical characteristics are shown in Table 1. Grades II–IV acute graft vs. host disease (aGVHD) was observed in 40 (36%) of the 111 patients and mild-to-moderate chronic graft vs. host disease (cGVHD) was observed in 27 (24%) patients.
General characteristics of patients receiving a haploidentical-related hematopoietic stem cell transplant and comparative analysis between patients with and without hemorrhagic cystitis (HC).
| No HC n = 81 | HC n = 30 | p | |
|---|---|---|---|
| Age in years, median (range) | 18 (1−63) | 17.5 (3−61) | 0.867 |
| Gender | |||
| Male | 53 (65.45%) | 19 (63.3%) | 0.837 |
| Female | 28 (34.6%) | 11 (36.6%) | |
| Pediatric patients, n= | 39 (48%) | 15 (50%) | 0.862 |
| Diagnosis, n= | |||
| Acute leukemia | 59 (72.8%) | 20 (66.7%) | |
| Lymphoma | 10 (12.3%) | 6 (20%) | 0.600 |
| Aplastic anemia | 9 (11.1%) | 2 (6.7%) | |
| Other | 3 (3.7%) | 2 (6.7%) | |
| Disease status at the time of transplant, n (%) | |||
| Complete remission | 41 (50.6%) | 13 (43.3%) | 0.573 |
| Active disease | 40 (49.4%) | 17 (56.6%) | |
| CD34+ cell/kg infused, median (range) | 9. 4 × 106 (1−20 × 106) | 7.75 × 106 (3−20 × 106) | 0.512 |
| Myeloid recovery, median time in days (range) | 17 (8−35) | 16 (11−25) | 0.463 |
| Platelet recovery, median time in days (range) | 16 (11−40) | 18 (11−30) | 0.637 |
| Acute Grades II-IV GVHD, n= | 27 (33%) | 13 (43.3%) | 0.401 |
| Chronic GVHD, n= | 19 (23%) | 8 (26.6%) | 0.804 |
| Mortality, n= | 40 (49%) | 16 (53.3%) | 0.712 |
Thirty (27%) of the 111 patients had HC, 19 (63.3%) males and 11 (36.6%) females, with a median age at transplantation of 17.5 (3–61 years). Fifteen (50%) patients were <18 years at the time of transplant. Indications for transplant included malignant disease in 26 (87%) patients and non-malignant disease in four patients. Grades II–IV aGVHD was observed in 13 (43.3%) patients, while eight (26.6%) patients developed cGVHD (Table 1). The skin was the most frequently affected site in both acute and chronic GVHD.
According to the HC classification, one patient (4%) was stratified at Grade I, 21 (70%) at Grade II, four (13%) at Grade III and four (13%) at Grade IV. The median time of HC onset was 30 days (7–149 days) post-transplant, with a median duration of 23.5 days (4–80 days). Late HC was observed in all 30 patients in this group. Using qPCR, BK virus levels were quantified in blood and urine samples from 14 patients; the BK virus was detected in 10 (71%) samples, nine in urine and one in blood, while in four samples, the BK virus was not detected. The median urinary viral load was 50,000,000 copies/µL (50,000,000–833,682,957 copies).
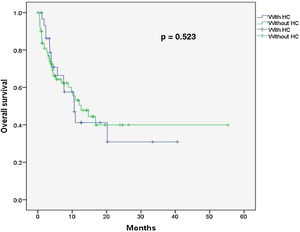
Due to financial constraints, BK virus detection was not performed in the remaining 16 patients of this group, thus the etiological HC agent in this subgroup was not identified. Urine cultures were performed for all 30 HC patients, but in all cases, cultures were negative for bacterial and fungal infection. The HC treatment was non-invasive in mild HC cases (Grades I–II) and invasive for severe HC or refractory cases. Non-invasive treatments were performed in an outpatient setting, with oral hydration, quinolone and pain relief in 16 (53%) patients. Fourteen (47%) patients required hospitalization for invasive treatment, which included Foley catheterization, intravenous hydration and pain relief. Additionally, one patient received intravenous cidofovir (5 mg/kg); four patients received intravesical cidofovir (5 mg/kg in saline solution, once a week for two weeks); three patients received continuous bladder irrigation (cystoclysis) with saline solution, using a two-way Foley urethral catheter; one patient needed cystostomy (suprapubic catheter insertion), with clot removal due to repetitive urinary obstruction; one patient required cystoscopy, with clot evacuation due to urinary obstruction, and; six patients required a blood transfusion. Quinolones comprised ciprofloxacin (500 mg, twice daily) or levofloxacin (500 mg, daily). Cidofovir was used for patients with detected BK virus and for those who did not response to quinolones. All patients responded to treatment, except two (6.6%), who died due to HC complications. One patient with cystostomy died due to sepsis, secondary to urinary tract infection and another patient with cystoclysis died due to renal failure and multiple organ failure, secondary to in-hospital pneumonia. In total, 16 (53.3%) patients in this group died, the main causes being relapse of underlying disease and infection. For patients without HC, the median follow-up was 6.6 months (1–41 months) and the overall survival was 46.7%, with no significant differences (p = 0.52) (Fig. 1). A significant correlation was observed between HC incidence and donor-receptor sex mismatch (r = 0.222, p = 0.02). However, no relationship was observed between the HC and patient age (r = −0.016, p = 0.86), total hematopoietic CD34+ cells infused (r = −0.063, p = 0.51), median post-transplant days to myeloid (r = −0.081, p = 0.46) and platelet recovery (r = −0.054, p = 0.63), presence of aGVHD (r = −0.081, p = 0.40) or cGVHD (r = −0.024, p = 0.80) and donor gender (r = −0.091, p = 0.34).
Characteristics of patients without HCEighty-one patients (73%) did not present with HC, 53 (65%) males and 28 (35%) females, with a median age at transplantation of 18 years (1–63 years). A total of 39 (48%) patients were <18 years. Indications for transplant included hematologic malignant disease in 69 (85%) cases and non-malignant disease in 12 (15%). At the time of transplantation, 41 (50.6%) patients were in complete remission and 40 (49.4%) had active disease. Grades II–IV aGVHD was observed in 27 (33%) patients. The median follow-up time was 4.8 months (0–55 months) and overall survival was 50%.
DiscussionIn this study, the HC incidence (27%) was similar to other studies, although reported ranges are wide3,6,22,31 In our outpatient HSCT program, hydration could be not efficiently delivered during the conditioning regimen and post-transplantation cyclophosphamide administration, favoring HC in our patients. To minimize the risk, we used Mesna and hydration, from commencement until six hours after the end of the cyclophosphamide infusion.
Many HC risk factors have been described, including the conditioning regimen, unrelated donor and patient characteristics, such as male gender, older age and clinical conditions, including GVHD.3,32,33 In our study, despite all patients having received an RIC and the hematopoietic cell source having been a related donor in all cases, the HC incidence was similar to previous reports on myeloablative regimens. However, we did not find any relationship between demographic characteristics and the HC incidence.
We observed that donor-recipient sex mismatch was more frequent in the HC group than in the group of patients without HC (60% vs. 41%). This sex mismatch correlated with higher HC incidences (r = 0.222, p = 0.02). The discrepancy between sexes was more frequent from male donors to female recipients (37% vs. 23%). Similar information was found in a previous study.34
Cyclophosphamide is used in haploidentical transplantation to induce T lymphocyte apoptosis, thereby reducing the risk of GVHD, without damaging key hematopoietic cells, which produces high levels of protective aldehyde dehydrogenase.35,36 At recommended post-transplantation cyclophosphamide doses of 50 mg/kg on days +3 and +4, previous studies showed a 6% incidence of Grade 3 aGVHD.13,37 In our study, the aGVHD incidence (36%) was higher than previously reported rates, but no correlations with HC incidence or severity (r = −0.081, p = 0.40) were observed.
Although the BK virus can be detected in the urine of 50%–100% of asymptomatic patients, not presenting HC, it is probable that the coexistence of BK virus with other factors could favor HC (6). It has been suggested that delayed immune reconstitution following haploidentical transplant could contribute to higher rates of BK virus infection in this population and that immune reconstitution leads to more severe BK virus infections, including HC incidences, due to the recognition of bladder viral antigens by emerging, functioning lymphocytes.38
Nevertheless, for associations between the BK virus and conditioning regimens, the BK viruria is more frequent in patients who receive a myeloablative conditioning regimen (57%), than those who receive a non-myeloablative scheme (30%), regardless of the presence of HC. In this study, we observed a high HC incidence associated with the BK virus (71%), bearing in mind all patients had received RIC schemes.6 Characteristically, late onset HC is associated with viral infection, mainly by the BK virus.31,39 All HC cases included here were late onset, with a median time after transplant of 30 days (7–149 days) and a median duration of 23.5 days (4–80 days). The BK virus was the most frequent etiological agent detected. Although virus detection was not performed in all patients due to onset characteristics (late onset) and financial constraints, it is unlikely that the HC was caused by the chemotherapy regimen in these cases. Regarding overall survival, we found no differences between patients with and without HC (46.7% vs. 50%, p = 0.712).
Study limitations included its retrospective nature, the small number of patients with HC and a possible underestimation of virus-associated HC, due to the low numbers of samples analyzed. However, our study provides key information on the HC incidence and evolution in patients who receive their haplo-HSCT on an outpatient basis, including all the care for the administration of the conditioning scheme and the post-transplant cyclophosphamide.
In conclusion, the HC incidence and severity after haplo-HSCT on an outpatient basis was comparable to incidences reported in hospitalized patients after myeloablative conditioning. The only characteristic related to a higher HC incidence was the donor-recipient sex mismatch.
Statement of ethicsAll transplant procedures were performed in accordance with institutional ethical standards after obtaining approval by the transplant committee and signing an informed consent, according to the FACT guidelines. Due to the retrospective nature of the study, it was not necessary to request informed consent from patients; however, patient confidentiality was strictly maintained.
Conflict of interestThe authors declare no conflicts of interest.