Hodgkin's lymphoma (HL) is a B-cell malignancy that affects 9000 new patients annually in the USA, representing approximately 11% of all lymphomas.1 In the last decades, randomized clinical trials conducted by cooperative groups in North America and Europe have identified treatment schedules that provide higher efficacy and lower toxicity with current treatment being expected to cure over 80% of patients.2 The three- and five-year progression-free survival (PFS) rates range from 82% to 94%3–5 in patients with localized disease and from 71% to 86% in patients with advanced disease.6–14
Autologous stem cell transplantation (auto-SCT) has become the standard of care for relapsed or refractory HL.15,16 It leads to long-term PFS rates of approximately 50% in relapsed patients and of 30–40% in patients with primary refractory HL.6,15–20
The treatment of relapsed or refractory HL patients with treatment failure after an auto-SCT is a therapeutic challenge. Disease relapse or progression after auto-SCT is associated with a poor prognosis, with a median overall survival of 2.4 years.21,22 While allogeneic stem cell transplantation (allo-SCT) represents the only potentially curative option, its role is still controversial.6,23,24 Data from retrospective and prospective studies support the use of allo-SCT in particular settings. Moreover, a recent analysis from the Center for International Blood and Marrow Transplant Research (CIBMTR) reported encouraging results with the use of reduced-intensity conditioning haploidentical transplantation, when compared to matched-unrelated donor transplantation.25 Briefly, when recommending the procedure, other aspects should be taken into account, such as age, the use of non-myeloablative approaches, donor factors (availability, type and matching) comorbidities and the substantial risks of treatment-related morbidities and mortality.6,23,24
For patients who are not candidates for allo-SCT, the goal of therapy is disease control with minimal toxicity.6,22,23,26,27 Multiple options are available in this setting, including single agent chemotherapy, combination chemotherapy, radiotherapy, antibody–drug conjugates, immune checkpoint inhibitors, immunomodulatory agents and small molecule inhibitors.
Overall response rates of single agents, including gemcitabine, etoposide, vinorelbine, liposomal doxorubicin, vinblastine and bendamustine, range from 22% to 72%, with complete response rates of 12% to 51% and median duration of responses ranging from 5 to 8 months.28–32 Several combination chemotherapy regimens based either on gemcitabine, platinum drugs or ifosfamide have been employed. Few data on the use of these regimens in the setting of relapse after auto-SCT are available, with overall response rates of approximately 70% and complete remission rates ranging from 19% to 50%.33–36 Most combination regimens have a high frequency of grade 3–4 myelosuppression.
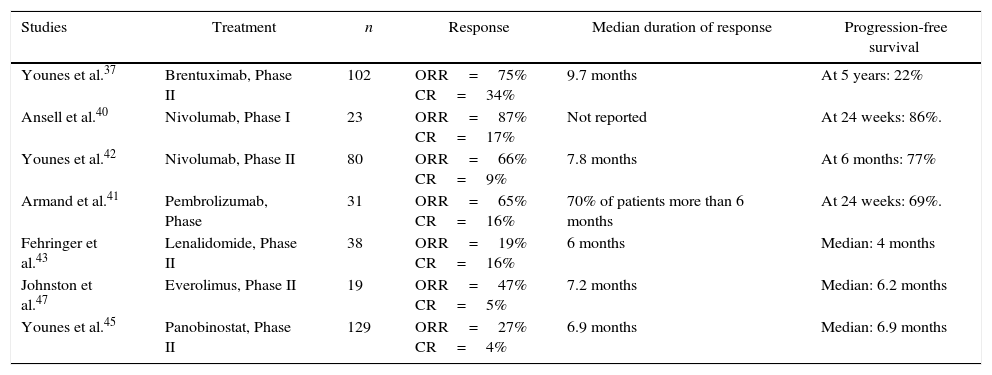
With the growing knowledge of HL biology, novel therapeutic agents aimed at specific molecular targets and pathways have been identified. Relevant published studies testing these agents in patients with active disease after auto-SCT are summarized in Table 1.23,26
Clinical studies with novel agents in patients with relapsed/refractory HL after autologous stem cell transplantation.
| Studies | Treatment | n | Response | Median duration of response | Progression-free survival |
|---|---|---|---|---|---|
| Younes et al.37 | Brentuximab, Phase II | 102 | ORR=75% CR=34% | 9.7 months | At 5 years: 22% |
| Ansell et al.40 | Nivolumab, Phase I | 23 | ORR=87% CR=17% | Not reported | At 24 weeks: 86%. |
| Younes et al.42 | Nivolumab, Phase II | 80 | ORR=66% CR=9% | 7.8 months | At 6 months: 77% |
| Armand et al.41 | Pembrolizumab, Phase | 31 | ORR=65% CR=16% | 70% of patients more than 6 months | At 24 weeks: 69%. |
| Fehringer et al.43 | Lenalidomide, Phase II | 38 | ORR=19% CR=16% | 6 months | Median: 4 months |
| Johnston et al.47 | Everolimus, Phase II | 19 | ORR=47% CR=5% | 7.2 months | Median: 6.2 months |
| Younes et al.45 | Panobinostat, Phase II | 129 | ORR=27% CR=4% | 6.9 months | Median: 6.9 months |
CR: complete response; ORR: overall response rate.
Brentuximab vedotin is an antibody–drug conjugate that selectively targets tumor cells expressing CD30. In the pivotal phase II multicenter trial of 102 patients treated for failure after an auto-SCT, 75% had a response, and 34% achieved complete remission (CR).37 At five years, overall survival (OS) was 41% and PFS was 22%. Patients who achieved a complete remission had superior outcomes (OS: 64%; PFS: 48%).38 Thirteen patients (38% of all CR patients) remained in remission. Among these 13 patients, four received a consolidative allo-SCT, and nine (9% of all enrolled patients) remain in CR without receiving any further treatment. Brentuximab was also tested as consolidation treatment after auto-SCT in patients with a high risk of relapse. In this study (AETHERA), patients given brentuximab had a median PFS of 42 months compared with 24 months in the placebo group.39
In recent years, the active role of neoplastic cells in downregulating the patient's immune response has been better understood. In HL, Reed-Sternberg cells express PD-1 ligands (PD-L1 and PD-L2), which interact with PD-1 expressed on activated T-cells, thus leading to tumor tolerance. Antibodies against PD1 and PD-L1 have been shown to hamper this downregulation in various types of cancer. Nivolumab and pembrolizumab, two monoclonal antibodies directed against PD-1, have shown good results in heavily pretreated patients with relapsed or refractory HL.40,41 In a phase Ib trial, 23 such patients received nivolumab until complete response, tumor progression, or excessive toxic effects. Most of the patients had been treated with an auto-SCT, and 78% had received brentuximab. An objective response was found in 87%, including 17% with a complete response. Progression-free survival at six months was 86%. Furthermore, the drug presented acceptable safety.40 These results led to a phase II trial of nivolumab in 80 patients with HL after failure of auto-SCT and brentuximab.42 An objective response was achieved in 66% of patients and 9% of complete remission. The median time to response was 2.1 months. Follow-up is ongoing to assess the long-term durability of nivolumab in this setting. Another study included 31 heavily pretreated HL patients treated with pembrolizumab. The overall response rate was 65%, with 16% in complete remission. Progression-free survival at 24 weeks was 69%.41
Lenalidomide has been tested in a few studies in patients with relapsed or refractory HL.43,44 In a phase II trial that included 38 patients with a median of four previous treatments, 87% of whom had received an auto-SCT, the objective overall response rate and complete response rate were 19% and 16%, respectively. Due to the acceptable toxicity profile, further studies to optimize doses and investigate the use of lenalidomide in combination with other drugs are needed.43
Drugs that target histone deacetylases may also be effective in HL. The most effective inhibitor of histone deacetylase in HL appears to be panobinostat. A phase II study with 129 HL patients treated previously with a median of four regimens was reported. Objective responses were achieved in 27%, including 30 (23%) partial responses and five (4%) complete responses. The median duration of response was 6.9 months and median PFS was 6.1 months.45
Signaling through the PI3-kinase/mTOR pathway has been demonstrated to be active in HL. Everolimus is an oral agent that specifically targets the mTOR complex1 (mTORC1).23,46 A phase II trial was conducted in nineteen patients with relapsed HL, who had received a median of six prior therapies, including 84% with a prior auto-SCT. The overall response rate was 47% and only one patient achieved a complete remission.47 In the current issue of the Brazilian Journal of Hematology and Hemotherapy, a retrospective analysis of 33 relapsed/refractory HL patients who received everolimus in a compassionate use program in Brazil is reported.48 Patients had received a median of five prior therapies and 88% had undergone an auto-SCT. The overall response rate was 45%; two (6%) patients achieved complete remission and 13 (39%) had a partial response. The median PFS and OS were 9 months and 36 months, respectively, somewhat similar to the findings in the previous phase II trial, in which the median PFS and OS were 6 months and 25 months, respectively. It is noteworthy that thirteen patients received treatment for more than one year, and three patients had been receiving it for more than four years, despite progression of the disease.
In summary, there is an ongoing effort to identify effective treatments for patients whose disease progresses after an auto-SCT. Drugs with innovative mechanisms, in particular brentuximab and nivolumab, have been quickly introduced into clinical practice in many countries. However, the therapeutic challenge is compounded by the high cost of these new drugs. The complexities of allo-SCT also limit its availability in the public health system. As better data on the long-term results of these novel treatments accumulate, we will hopefully, be able to better define the best approaches, and offer them to every patient in need.
Conflicts of interestThe authors declare no conflicts of interest.
See paper by Rocha et al. on pages 216–22.