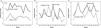The date of acute lymphoblastic leukemia (ALL) diagnosis has been studied regarding potential etiologic roles with contrasting results and the issue remains controversial. The principal aim of this study was to analyze monthly variation of ALL diagnosis in a large homogenous Hispanic Latin American cohort over 15 years; its association with survival rates was also assessed.
MethodsClinical files and electronic records of 501 consecutive patients of all ages with ALL in northeastern Mexico over the years of 2004–2018 were scrutinized. Patients were divided into children ≤18 and adults >18 years. The Chi–square heterogeneity analysis was used to test for non-uniform variation. The Poisson regression analysis was used to fit sinusoidal (harmonic) models to the data, using the month of diagnosis as a covariate in a separate model.
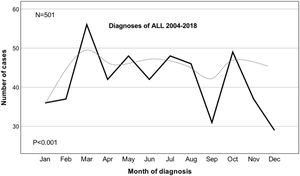
ResultsDuring the study period 363 children (72.5%) and 138 adults (27.5%) (p < 0.001) were diagnosed with ALL. Heterogeneity across the months of diagnosis was confirmed (p = 0.019) and the Poisson regression analysis confirmed a significant monthly variation (p < 0.001) (95% CI, 3.024–3.745), a higher annual peak being observed in the month of March (p = 0.002), followed by a second peak in October (p = 0.026). The five-year OS for children was 68.2% (95% CI, 67.64–68.74) and for adults, 43.7% (95% CI, 42.67–44.71) (p < 0.001). No significant association between the month of diagnosis and OS was found (p = 0.789).
ConclusionThe monthly variation of ALL diagnosis was documented; these results confirm the heterogeneous behavior of the disease and appear to be consistent with an interplay of environmental and biologic factors. Further studies are needed to examine putative candidate agents.
Acute lymphoblastic leukemia (ALL) represents the most common childhood malignancy, accounting for approximately 28% of all cancers and 80% of all leukemias in children and comprising <1% of adult cancers.1,2 The ALL presentation is bimodal, with the first peak occurring in childhood and a second peak, around the age of fifty.1 Its incidence is reported to be higher in Hispanic children, at 43 cases per million, vs. 28 in non-Hispanics.3
While the pathogenesis of ALL is complex and remains elusive, exogenous and/or endogenous exposures, genetic susceptibility, biologic heterogeneity and chance appear to play a role. Environmental risk factors, including infections, are of particular interest in the etiology of leukemia and epidemiological investigation into its onset periodicity, leukemia being a disease with a long history, as well as the evidence for seasonal variation in its incidence, may provide insight into this hypothesis. Many viral infections have characteristic temporal onsets, thus investigations had suggested that ALL is related to infection or other seasonally varying environmental risk factors.4–6
Time trends in the diagnosis of ALL may provide evidence of an infectious etiology, as seasonal climatic changes give rise to respiratory infections or gastrointestinal infections in winter, spring and summer. Moreover, these could suggest the presence of environmental factors such as pesticides, which are applied frequently in rural areas. Also, spatiotemporal clusters occur, with an excess of ALL cases observed in a determined geographical area at certain points in time, compared to other areas and other times.7 Importantly, most studies reporting temporal trends do so in countries with temperate-cool climates,8 with a few conducted in arid or semiarid regions, which have a rather short winter season.
Studies addressing temporal trends of acute leukemia presentation are scarce and report contrasting results.9–11 Time trends and seasonal variations have been assessed and while some reports have not identified a seasonal influence,11,12 others have found a significant temporal pattern.10,13,14
Gao et al. hypothesized that in those populations in which ALL seasonality occurred and arose from a climatic determinant, one would expect to find a more evident and pronounced seasonality effect as the latitude of the population studied moved away from the equator; after analyzing reports from several countries including South Africa, Singapore, Iran, Russia, Sweden and the USA, among others, he did not find this pattern and rejected the hypothesis.10 Most studies on this subject have analyzed different reference dates, including those of birth, first symptom, or diagnosis, and have used different statistical methods, leading to inconsistencies and controversy in the results. England and Wales have documented seasonality of ALL in summer,15 meanwhile Italy and Denmark have reported it in the winter season;8,16 suggesting that this variation may be the result of a relationship between leukemia and infectious processes or between leukemia and some other phenomenon that undergoes a temporal variation.
We hypothesized that the presentation of ALL cases would tend to occur in specific months, rather than in one season. The monthly variation of ALL diagnosis in a homogeneous group of Hispanic children and adults over a 15-year period and their survival rates were studied.
MethodsStudy populationA fifteen-year observational, longitudinal and retrospective analysis was performed from 2004 to 2018 on patients of all ages with a diagnosis of ALL, classified according to the World Health Organization, at the Hematology Department of the Dr. Jose Eleuterio Gonzalez University Hospital of the School of Medicine at the Universidad Autonoma de Nuevo Leon in Monterrey, Mexico. The region is semiarid and has a latitude of 25°40′17″ North and longitude of 100°18′31″ West.
We analyzed the clinical hard-copy and electronic files of 501 patients and documented their age, sex, white blood cell (WBC) count at diagnosis, disease immunophenotype, risk stratification, therapy received, date of diagnosis and date of last visit or death. For the purposes of this study, we considered the date of diagnosis as the date of the first pathological data registered and only included in the study consecutive patients with a symptom-diagnosis interval <4 weeks to minimize the bias generated due to the delayed presentation in the monthly variation. The objective of the present report was to study the variation of ALL cases presented per month of the year, instead of expecting a seasonal peak. For a further stratified analysis, patients were classified by age, considering children those <18 years and adults, those >18 years of age, based on the Children's Oncology Group approach and the National Comprehensive Cancer Network 2020 Guidelines. No patient was excluded due to lack of data. The Institutional Ethics and Research Committee approved the study protocol and waived informed consent due to its retrospective methodology.
Treatment for childrenChildren were stratified into standard- and high-risk groups17 to receive risk-adapted chemotherapy. Patients received two protocols designed at our center, based on drug availability, the first one between 2004 and 2009. After 2010, the treatment protocol changed to another Berlin-Frankfurt-Münster (BFM)-based scheme. Both protocols were administered as described elsewhere.18
Treatment for adultsPatients received BFM-modified chemotherapy, including three or four drugs, according to a protocol previously reported for adults. Details of drugs and dosages administered have been reported.19
Statistical analysisPoisson regression models were used to test for monthly heterogeneity in ALL diagnosis. This method fits sinusoidal (harmonic) models to the data, using observed counts as the outcome and expected counts and month of diagnosis as covariates. The significance of the sinusoidal model assumption was evaluated with post-estimation Pearson Chi–square tests for goodness-of-fit. The overall temporal trend was also tested applying a Poisson regression model. All models were evaluated for significant trends, using post-estimation statistics. Additionally, the Chi–square test of homogeneity to test the hypothesis of no difference in the number of ALL diagnoses by month was performed. Transplanted patients were included as censored cases; the overall survival (OS) was measured from the time of diagnosis to the time of transplant, time of death or last visit. The Kaplan–Meier method was employed to estimate the OS and compared, using the log-rank test. The Cox proportional hazard regression model was used to identify the multivariate risk factors for the OS. For the multivariate analysis, stepwise analysis was used with a variable entry criterion of p < 0.05 and a variable retention criterion of p < 0.05. Cox analyses were summarized as the hazard ratio (HR), 95% confidence interval (CI) for the HR and the corresponding p-value. The SPSS v.26 (IBM SPSS Statistics, IBM Corporation, Armonk, NY) was used for data analysis. A p-value < 0.05 was considered statistically significant.
ResultsDescriptive characteristics of patients with ALL are displayed in Table 1. There was a total of 501 ALL cases diagnosed in patients of all ages during the period 2004–2018 at our center. There were 279 (55.7%) males and 222 (44.3%) females, with a male-to-female ratio of 1.26 (p = 0.011). There were 363 children (72.5%) and 138 adults (27.5%) (p < 0.001). For the whole group, the median age at diagnosis was 11 years (2 months–85 years); the median age for females was 8 years (2 months–78 years) vs. 12 years (3 months–85 years) for males (p = 0.009). The median follow-up was 32 months (1–171 months). The overall Chi–square heterogeneity test showed a statistical evidence of departure from the uniform distribution for the month of diagnosis (x2 = 22.62, p = 0.019). Subsequently, the Poisson regression modelling confirmed a significant monthly variation (p < 0.001) (95% CI, 3.024–3.745). As observed in Fig. 1, the months with the maximum number of cases were March (p = 0.002) and October (p = 0.026).
Principal characteristics of an acute lymphoblastic leukemia cohort of 501 patients treated at a single reference center from 2004 to 2018 in northeastern Mexico.
| Characteristic | Age group | |
|---|---|---|
| Children ≤18 years | Adults >18 years | |
| n = 363 (72.5%) | n = 138 (27.5%) | |
| Median age (years), range | 6 (0.2–18) | 32 (19–85) |
| Median follow-up (months), range | 43 (1–171) | 22 (1–165) |
| Sex | ||
| Male | 199 (54.8%) | 80 (58%) |
| Female | 164 (45.2%) | 58 (42%) |
| Status | ||
| Dead | 110 (30.3%) | 62 (44.9%) |
| Alive | 253 (69.7%) | 76 (55.1%) |
| Immunophenotype | ||
| T | 20 (5.5%) | 6 (4.4%) |
| B | 343 (94.5%) | 132 (95.6%) |
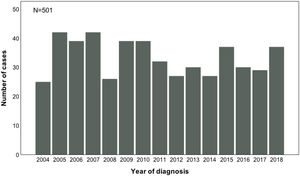
In Fig. 2, the presentation of ALL cases through the years of 2004–2018 showed a heterogeneous distribution (p = 0.179). The years with the maximum number of cases were 2005 and 2007, with 42 new cases each, whereas 2004 was the year with the lowest number, twenty-five.
ChildrenChildren represented the majority of the cohort; there were 199 males (54.8%) and 164 females (45.2%), (p = 0.066). The median age at diagnosis was 6 years (2 months–18 years). The Chi–square heterogeneity test showed a statistical evidence of departure from the uniform distribution for month of diagnosis (x2 = 21.44, p = 0.029). The Poisson regression modelling confirmed a significant monthly variation (p < 0.001) (95% CI, 2.753–3.561). A graph of monthly ALL diagnoses stratified by age group is presented in Fig. 3A, the three months of maximum cases in children being March (10.7%), May (10.5%) and October (9.9%), adding up to 31.1% of the childhood diagnoses. The lowest numbers of children ALL cases presented in September (5.5%), December (6.3%) and February (6.9%). In males, three main peaks were found in July (13%), October (11.5%) and March (11%), adding up to 35.5%. Females had only one main peak in May, accounting for 15.2% (Fig. 3B).
(A) Monthly variation in diagnosis of 501 patients with acute lymphoblastic leukemia (ALL) between 2004 and 2018 stratified by age group. (B) Monthly variation in diagnosis of 363 children with ALL between 2004 and 2018 stratified by sex. (C) Variation in month of diagnosis for 138 adults with ALL between 2004 and 2018 stratified by sex.
There were 80 males (58%) and 58 females (42%), (p = 0.061), in the study. The median age at diagnosis was 32 years (19–85 years). The Chi–square heterogeneity test was performed (x2 = 9.61, p = 0.565). The Poisson regression modelling found a significant monthly variation (p = 0.001) (95% CI, 1.103–2.641). The three months of maximum incidence in patients >18 years were March (14.5% of all adults), July (10.1%) and August (10.1%), adding up to 34.7%, while those of minimum incidence were January (4.3%) and December (4.3%). As shown in Fig. 3C, adult men and women shared March as a peak, July and October being months with a high number of cases for men, as well. Women presented a second peak in August.
SurvivalFor all 501 patients, the five-year OS was 60.5% (95% CI, 59.98–61.01). The five-year OS for children was 68.2% (95% CI, 67.64–68.74) and for adults, 43.7% (95% CI, 42.67–44.71) (p < 0.001) (Fig. 4A). For males, the five-year OS was 54.8% (95% CI, 54.11–55.48) and for females, 68.1% (95% CI, 67.36–68.81) (p = 0.044) (Fig. 4B). The difference in the OS of patients stratified by month of diagnosis showed no statistical significance (p = 0.789). Similarly, when patients diagnosed in the first half of the year vs. those diagnosed in the second half of the year were compared, no significant difference was observed. For patients diagnosed from January to June, the five-year OS was 65.1% (95% CI, 64.42–65.76) and for those diagnosed from July to December, 54.8% (95% CI, 54.03–55.56) (p = 0.063) (Fig. 4C).
Survival for childrenFor male children, the five-year OS was 63.2% (95% CI, 62.43–63.95), while for female children, it was 74.4% (95% CI, 73.64–75.13) (p = 0.181). For children with T-cell ALL, the five-year OS was 45.3% (95% CI, 42.85–47.71) and for those with B-cell ALL, 69.6% (95% CI, 69.04–70.14) (p = 0.043).
As shown in Table 2, in the multivariate Cox regression analyses, age (HR = 1.92, p = 0.044) was significantly associated with a decreased OS for children treated between 2004 and 2009, while risk (HR = 3.48, p = 0.004) was associated with a decreased OS for children treated after 2010.
Multivariate Cox Proportional Regression Analysis of 363 children with acute lymphoblastic leukemia according to treatment period in a single reference center from 2004 to 2018 in northeastern Mexico.
| Characteristic | Overall survival | |||
|---|---|---|---|---|
| Multivariate | ||||
| 2004–2009 | 2010–2018 | |||
| (n = 132) | (n = 231) | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age at diagnosis (<1 or ≥10 years) | 1.92 (1.02–3.64) | 0.044 | 1.81 (0.96–3.14) | 0.067 |
| Gender (male) | 1.48 (0.75–2.91) | 0.253 | 1.19 (0.69–2.05) | 0.515 |
| WBC count at diagnosis (≥50 × 109/L) | 1.37 (0.60–3.13) | 0.447 | 1.41 (0.81–2.44) | 0.215 |
| Immunophenotype (T-cell) | 2.55 (0.89–7.27) | 0.079 | 1.52 (0.53–4.33) | 0.433 |
| Risk (High) | – | – | 3.48 (1.47–8.23) | 0.004 |
| Diagnosed from Jan–Jun vs. Jul–Dec | 1.54 (0.79–3.01) | 0.197 | 1.71 (0.97–3.01) | 0.062 |
WBC: white blood cell, Jan–Jun: January to June, Jul–Dec: July to December.
For adult males, the five-year OS was 38.4% (95% CI, 37.14–39.65), while for adult females, it was 51.8% (95% CI, 50.10–53.47), (p = 0.165). For adults with T-cell ALL, the five-year OS was 20.8% (95% CI, 17.31–24.51), while for those with B-cell ALL, it was 44.9% (95% CI, 43.85–45.93), (p = 0.230).
The Cox regression analysis was performed for adults (Table 3). A WBC count ≥100 × 109/L at diagnosis (HR = 3.64, p = 0.001) and risk group (HR = 3.92, p = 0.001) were significantly associated with a worse OS.
Multivariate Cox Proportional Regression Analysis of 138 adults with acute lymphoblastic leukemia treated at a single reference center from 2004 to 2018 in northeastern Mexico.
| Characteristic | Overall survival | |
|---|---|---|
| Multivariate | ||
| HR (95% CI) | p-value | |
| Age at diagnosis (≥35 years) | 1.28 (0.63–2.59) | 0.488 |
| Gender (male) | 1.38 (0.72–2.64) | 0.326 |
| WBC count at diagnosis (≥100 × 109/L) | 3.64 (1.85–7.18) | 0.001 |
| Immunophenotype (T-cell) | 1.58 (0.45–5.56) | 0.469 |
| Risk (High) | 3.92 (1.38–11.15) | 0.001 |
| Diagnosed from Jan–Jun vs. Jul–Dec | 1.36 (0.65–2.87) | 0.410 |
WBC: white blood cell, Jan–Jun: January to June, Jul–Dec: July to December.
Contemporary analyses of a homogeneous ethnic and socioeconomic group of children and adults at a single reference center in a low-middle income country over a fifteen-year period provide evidence for a significant trend per month of the ALL diagnosis, with the highest number of cases diagnosed in March, followed by October.
Despite the fact that seasonality may provide insights into etiological risk factors, such as infectious agents, environmental exposure and variations in host immune responses, it has been investigated inconsistently in a variety of malignancies. The present report assessed the monthly variation of the ALL diagnosis and found it significant for all 501 patients as a group, as well as for children and adults independently. Similarly, a recent single-center report on seasonal variations in the diagnosis of hematological disorders, observed a significant variation in their ALL patients.20 Furthermore, a large 18-year French study evaluated time trends and seasonal variations in the diagnosis of childhood ALL, the seasonal variation only being documented in 1–6-year-old males, with an increase in the incidence in April, August and December.21 On the contrary, the only study that analyzed seasonality of the diagnosis of acute leukemia in a Hispanic population in Latin America included both ALL and acute myeloblastic leukemia (AML) and did not document a temporal pattern of presentation;11 a similar report in a Middle Eastern country also did not find a seasonal effect related to the month of diagnosis in adult acute leukemias (also including both ALL and AML).12 In the last two reports mentioned, patients with AML were included in the analysis and a reduced number of years was assessed, these limitations possibly explaining in part the lack of significant findings.
In 1934 Lambin and Gerard, the pioneers of studies to establish a relationship between leukemia and the season of year, concluded that there was a peak incidence from November to February in the Belgian population.14 Other studies highlighted the elevated incidence during the winter months;8,16 but in England and Wales, the largest number of ALL cases occurred in the summer.15 Additionally, one study conducted in Southern China including patients of all ages analyzed the monthly distribution for the onset of ALL symptoms and found a peak in July.22 In contrast to these findings, a review of twenty-four reports from eleven countries at locations ranging from 35.05° South to 65.0° North did not find a consistent pattern of seasonality.10
The precise etiology of ALL has not been established and only exposure to radiation in utero and Down syndrome have been recognized as risk factors associated with this malignancy; yet, these account for a very small part of the cases.23 Environmental risk factors, including infections, radiation, electric energy sources and other causes have long been suspected to play an important role in the development of the disease.24 A multi-stage two-hit model has been proposed with the first hit of genetic susceptibility possibly occurring before conception or in the pre-natal stage and the second hit ensuing in the extrauterine life through environmental exposure.25 Previous reports suggest that antigenic stimulation after community-acquired infections may activate oncogenes or disrupt tumor suppressor genes26,27 and thus, temporal patterns in the incidence of ALL in the context of an infectious etiology have been studied, with mixed results.28 Our findings document the existence of a monthly pattern and appear to support the putative role of an environmental factor (s), as a second hit influencing ALL development in our Hispanic cohort.
In both, the Chi–square test and Poisson regression analysis, a significant monthly variation was found in children, documenting March as the month with the highest number of cases. As in our report, another study assessing seasonal variation in the diagnosis of pediatric ALL documented March as their peak month.29 Secondarily, we aimed to report the survival rates of the study population; as expected, children showed a higher OS than adults and a decreased survival for males, when compared to females, was confirmed. Additionally, the month of diagnosis did not show a significant association with the OS; this last finding is not conclusive, owing to the small number of events in our 15-year cohort, which is a limitation in the making of meaningful analyses regarding the influence of a specific month. Finally, no significant association was found between the semester of the diagnosis (January–June vs. July–December) and the OS, when it was assessed with Kaplan–Meier and multivariate Cox analyses. These findings are in contrast to another study in which a significantly decreased OS was reported for patients diagnosed in the winter season.30
Regarding the incidence of ALL throughout the fifteen years of the study at our center, the variation observed could be partially explained by the inconsistency of the access that the population had to social security health services during the study period. Our center is a public institution that provides medical care to the open population of uninsured patients and in our country social security coverage depends on having a formal, permanent job, which is why, depending on having or not formal employment, families often have variable access to medical care; therefore, this may have influenced the annual presentation of ALL.
Evidence of the ALL monthly variation was present in our fifteen-year analysis of 501 consecutive patients. These data need to be interpreted considering several limitations, one of which was that the study was conducted at a single public center. As in other reports, the case group is a selected series derived from admission or clinical visits to an institution, rather than a population-based identification of all incidence cases; the chance of a variable referral pattern to other centers remains. Socioeconomic and sociocultural factors must be considered, since health insurance coverage in the study population depends on the beneficiaries having a formal job and thus, some patients may not have access during some months of the year, but may have access during other times of the year; therefore, the fluctuation of the ALL presentation may be partially influenced by a referral bias. Despite these limitations, the design of our study and inclusion of all pediatric and adult ALL cases over a 15-year period provides valuable information.
ConclusionGiven the potential for confounders, it is challenging to conclude that ALL has an infectious etiology; however, the presence of one or more temporal-varying environmental risk factors is highly plausible as a significant monthly variation was documented. The temporal trend of a putative agent could vary between regions and time periods, it could be specific to a leukemia subtype and a particular age or gender group, which might partially explain the inconsistencies in the existing reports. Future investigation of specific temporal-varying risk factors is necessary to explain the cause for the temporal variation observed in the diagnosis of ALL in the study population.
Conflict of interestThe authors declare no conflicts of interest.
We thank Sergio Lozano-Rodriguez, M.D., for his help in editing the manuscript.