Venous thromboembolism (VTE) constitutes the leading cause of maternal mortality in the developed world.1-4 The incidence of confirmed VTE during pregnancy and puerperium ranges from 0.5 to 3.0 per 1,000 pregnancies, representing 5- to 10-fold increased risk when compared with age-matched non-pregnant women.3,5 Pelvic vein thrombosis (PVT) is an important cause of VTE in the post-partum period, although few studies have investigated its incidence and clinical relevance.6,7 Furthermore, studies of VTE in the obstetrical population in the developing world are scarce8 or missing, in the case of PVT. The aim of this study was to evaluate the role of magnetic resonance venography (MRV) to detect PVT in women after Cesarean (C)-section delivery. To investigate this, we performed pelvic MRV within seven days after delivery and followed women in the postpartum period.
This was a prospective cohort study. The inclusion criteria were women older than 18 years, who underwent C-section delivery at the Obstetrical Unit of University Hospital, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil. The exclusion criteria consisted of women who received anticoagulation during pregnancy, had contraindications to MRV and/or who were critically ill. The study was approved by local research Ethical Committee.
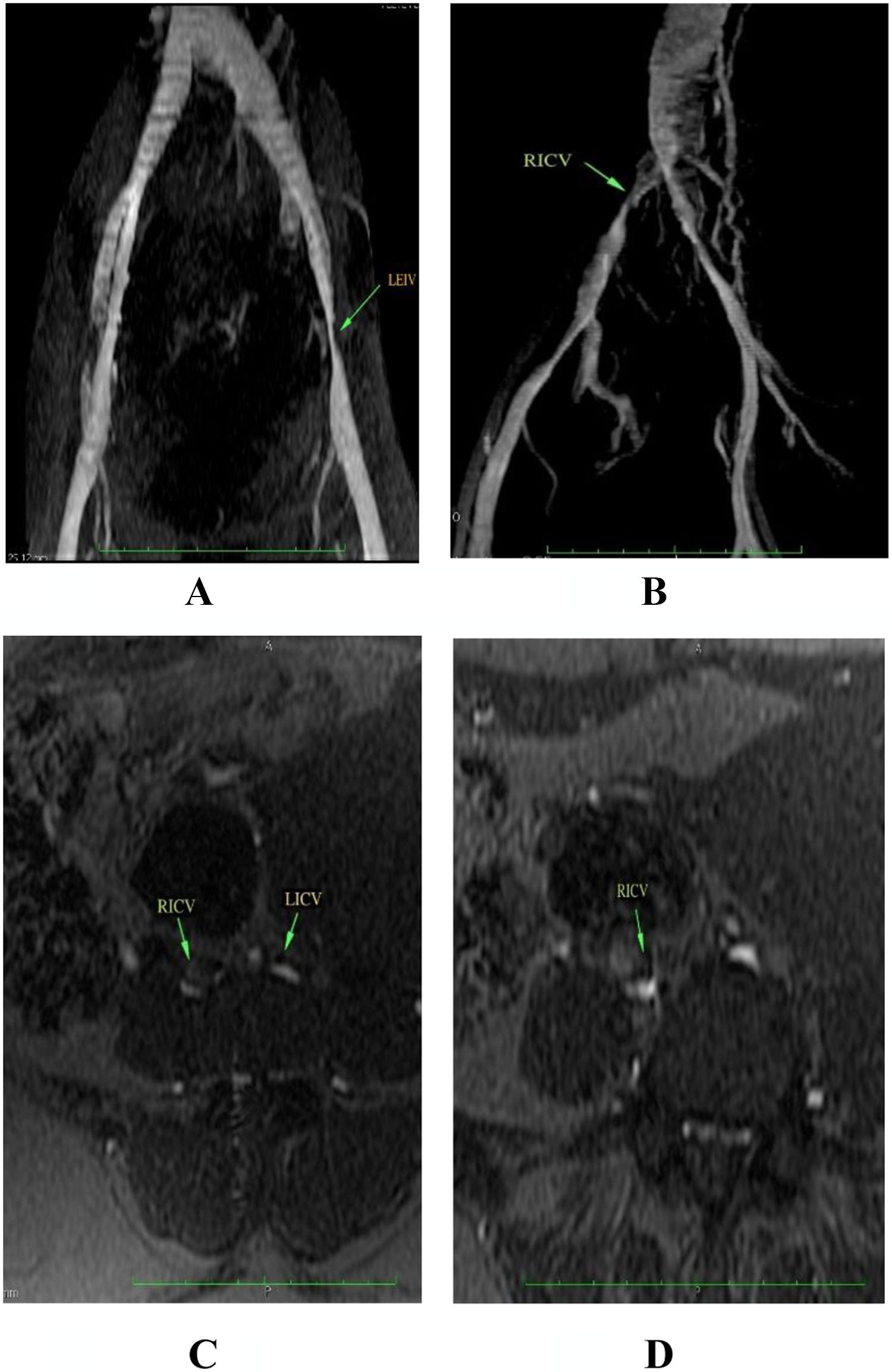
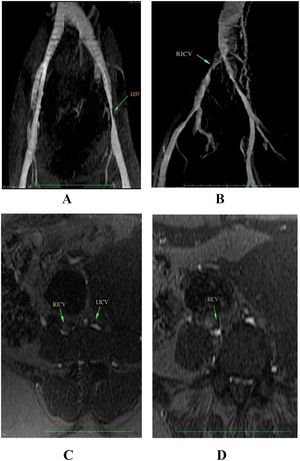
We collected information on socio-demographic, clinical data and risk factors for VTE during interview and from medical charts using a structured questionnaire. Women were included consecutively and followed by telephone interview every 30 days until 100 days post-partum. All women underwent a pelvic MRV, which was performed between day 0 and 7 after delivery. Pelvic MRV was performed on a 1.5-T scanner without contrast (Signa Excite, General Electric, Milwaukee, USA). The sequences consisted of gradient-echo (FIESTA) in axial/coronal plans and a 3D Time of Flight (TOF) angiography with arterial flow suppression and maximum intensity projection (MIP) reconstructions for assessment of vein patency. We defined PV as external iliac veins, internal iliac veins, common iliac veins, ovarian veins and inferior vena cava. Suggestive PVT was considered when there were filling defects, presence of collateral veins, vessel irregularities or vessel narrowing. The presence of venous enlargement and signs of perivascular inflammation suggested recent thrombus. Two experienced radiologists blindly and independently adjudicated MRV and disagreements were resolved by a third observer. Women presenting filling defects on MRV repeated it after 12 weeks.
A total of 64 women were included, of whom 14 (22%) withdrew the informed consent after inclusion but before the performance of MRV. The final cohort comprised 50 women, median age 26 years (interquartile range [IQR], 22-32) (Table 1).
Characteristics of the subjects included in the study.
N, number; IQR, interquartil range; VTE, venous thromboembolism; CPOD, chronic pulmonary obstructive disease.
Median age of excluded group was 26 years (IQR, 20 - 33). A total of 4/50 women (8.0%; 95% CI, 3.2 % - 18.8 %) had MRV findings suggestive of filling defects in the postpartum period of whom 1 had no risk factor for VTE and 3 had one risk factor each (Figure 1, Table 2). The location of the potential filling defect varied (Table 2). All filling defects were semi-occlusive. All women were asymptomatic and none received anticoagulation. Agreement rate between the two radiologists was 100%.
Characteristics of the subjects presenting with filling defects by magnetic resonance venography of the pelvic veins.
The green arrows show filling defects in the pelvic veins.
RICV, Right Iliac Common Vein; LICV, Left Iliac Common Vein; LEIV, Left External Iliac Vein. Coronal (A and B), Axial (C and D)
A total of 49 out of 50 women (98%) were followed by telephone interview at 30, 60 and 90 days. The totality of women without filling defects by MRV did not report any complications during follow-up. The four subjects presenting with filling defects repeated the MRV at 116 –126 days after the first MRV showing no filling defects in the PV. They reported no symptoms/signs of VTE or other complications during the follow-up.
Few studies have investigated PVT after C-section7 and post-vaginal delivery.6 Both studies revealed a high rate of definitive PVT by MRV after C-section (46 %) in women with moderate to high risk for VTE7 and post-vaginal delivery (30 %) in women with low risk for VTE.6 In corroboration with our study, none of the thrombi was occlusive in the study by Roger et al.7 However, these authors did not report follow-up of included women or repetition of the MRV in women with suspected PVT. To our concern, this is the first study reporting follow-up of women with suspected PVT after C-section.
In contrast with reported studies,6,7 we found filling defects in the PV in a less proportion of women after C-section delivery (8%) and none developed symptomatic PVT or other form of VTE. The encountered filling defects disappeared during the follow-up. These results suggest that either semi-occlusive PVT might be a thrombotic condition without clinical consequence,9 such as it is in the case of distal DVT of the legs or that the filling defects identified are artefactual due to extravascular compression mainly by an increased uterine volume, anatomical distortions, or other image defect which could resemble PVT. James has also suggested that thrombosis of PV could be part of a physiologic process to interrupt blood flow at the placental site and facilitate placental involution.9 However, we consider that this is a strong statement to define those filling defects as thrombi.
Our study poses limitations worth mentioning. Firstly, participants included in our study may not represent the overall population of pregnant women, once our hospital has a reference obstetrical unit for high-risk pregnancies. This could, therefore, introduced a selection bias. Secondly, since the study has been performed in one hospital, this reduces the generalizability of our findings.
In conclusion, we found filling defects in the PV in 8% of women after C-section delivery and none developed symptomatic PVT or other form of VTE. Further studies should be directed towards establishing baseline MRV findings in the immediate postpartum period in larger cohorts of women, to establish better estimates of the true population incidence of PVT, assess its natural history and to delineate how long filling defects (or thrombi) remain visible in the pelvis after delivery.
The authors thank the participants, MRI technologists, radiologists and the nursing team of the Obstetrics Unit, University Hospital, Universidade Federal de Minas Gerais.
This study was supported by FAPEMIG, grant number EFP00001296 and CNPq (PIBIC)