Lipoprotein apheresis (LA) is an extracorporeal therapy which removes apolipoprotein B-containing particles from the circulation. We evaluated techniques and efficiency of lipoprotein apheresis procedures applied to patients with familial and non-familial hypercholesterolemia (FH) at our center.
MethodsWe retrospectively evaluated 250 LA procedures applied to 27 patients with dyslipidemia between March 2011 and August 2019.
ResultsA total of 27 patients, of whom 19 (70.4%) were male and 8 (29.6%), female, were included. Eighteen (66.7%), 6 (22.2%) and 3 (11.1%) patients were diagnosed with non-FH, homozygous FH (HoFH) and heterozygous FH (HeFH), respectively. Two different apheresis techniques, direct adsorption of lipoproteins (DALI) (48.8%) and double filtration plasmapheresis (DFPP) (51.2%), were used. The change in the serum total cholesterol (TC) level was the median 302 mg/dl (171–604 mg/dl) (60.4%) in HoFH patients, 305 mg/dl (194–393 mg/dl) (60.8%) in HeFH patients and 227 mg/dl (75–749 mg/dl) (65.3%) in non-FH patients. The change in the serum low-density lipoprotein (LDL) level was the median 275 mg/dl (109–519 mg/dl) (64.2%), 232 mg/dl (207–291 mg/dl) (64.5%) and 325 mg/dl (22–735 mg/dl) (70.9%) in patients with HoFH, HeFH and non-FH, respectively. A significantly effective reduction in serum lipid levels, including TC, LDL and triglycerides, was achieved in all patients, regardless of the technique, p < .001. The decrease in the serum TC and LDL levels was significantly higher in the DFPP, compared to the DALI, being 220 mg/dl (−300 to 771) vs 184 mg/dl (64–415), p < .001 and 196 mg/dl (11–712) vs 157 mg/dl (54–340), p < .001, respectively.
ConclusionsOur results showed that LA is a highly effective treatment in reducing serum lipid levels and safe, without any major adverse event.
Severe hypercholesterolemia is a well-known risk factor for coronary heart disease and familial hypercholesterolemia (FH) is one of the most common inherited diseases leading to significant cardiovascular (CV) mortality and morbidity. In patients with heterozygous familial hypercholesterolemia (HeFH), the untreated cholesterol levels typically range between 250 and 300 mg/dl and cardiovascular events occur in women by 40–60 years of age and in men by 30–50 years of age.1–4 In patients with homozygous familial hypercholesterolemia (HoFH), severe atherosclerotic events begin from early childhood due to much higher serum cholesterol levels (500–1000 mg/dl). Early initiation of treatment in these patients is crucial because homozygous individuals generally die before the age of 30 years due to accelerated atherosclerotic events, if left untreated.5
Lipoprotein apheresis (LA) is a well-established extracorporeal technique, in which apoB-containing lipoproteins are selectively removed from the plasma of a patients.6,7 The reduction in serum Lp(a) and low-density lipoprotein (LDL) levels may have a beneficial effect on patients with HoFH, severe HeFH with progressive cardiovascular disease and some patients with hyper-lipoproteinemia(a) [hyper Lp(a)]. Despite advances in the pharmacotherapy of lipid disorders, many patients have failed to achieve satisfactory lipid-lowering effects to reduce cardiovascular disease (CVD). LA contributes to reducing the risk of cardiovascular events in these patients.8,9 Previous studies revealed that LA is beneficial to the radical modification of atherogenic lipoproteins for the reduction in cardiovascular events.10–12 The European Atherosclerosis Society (EAS) recommends that healthcare providers consider LA for patients with progressive coronary disease and Lp(a) >600 mg/l and whose serum LDL levels remain >3.2 mmol/l despite maximal drug therapy.13 These suggestions are currently under review to take into account the newer pharmacological treatments and the lower target levels of LDL cholesterol proposed by the EAS.
Severe hypertriglyceridemia (>10 mmol/l, or >900 mg/dl) may cause acute pancreatitis due to increased levels of very low-density lipoprotein (VLDL), chylomicrons and remnant particles. Therapeutic plasmapheresis with a centrifugal cell separator enables triglyceride levels to be drastically reduced with a rapid resolution of symptoms in the acute phase of the disease.14 Chang et al. showed that treatment with double filtration plasmapheresis (DFPP) in patients with extreme hypertriglyceridemia and acute pancreatitis shortens the duration of hospitalization, when compared to patients receiving conventional therapy.15
In the present study, we retrospectively evaluated patients with hyperlipidemia, including HoFH, HeFH and non-familial hyperlipidemia (non-FH), who underwent lipoprotein apheresis at our center. In addition to the lipid lowering effects of the LA procedures, we also investigated the data including the apheresis technique, duration of procedure and procedure-related complications, if they occurred.
MethodsPatientsBetween March 2011 and August 2019, 27 patients with HoFH, HeFH and non-FH had undergone LA procedures at our center and all were included in the study. Medical records were retrospectively reviewed in terms of gender, age, height, weight, body mass index (BMI) and the main indication for LA. Serum lipid levels were evaluated before and after each LA session. Cardiovascular disease risk factors, comorbidities and the lipid-modifying drugs administered were also recorded. The study was approved by the local ethics committee.
Apheresis procedureTherapeutic plasmapheresis is an extracorporeal blood purification technique designed for the removal of pathogenic substances from the plasma of patients, including pathogenic autoantibodies, immune complexes, cryoglobulins and cholesterol-containing lipoproteins.16 At our center, the LA techniques used were double filtration plasmapheresis (DFPP) and direct adsorption of lipoproteins (DALI). Therapeutic plasmapheresis devices purchased from commercial companies named AsahiKASEI®, Kaneka®, Informed® and Medica® were built upon the DFPP technique, whereas 4008 ADS (Fresenius HemoCare Adsorber Technology®, GmbH, St. Wendel, Germany) used the DALI technique. All LA procedures were reviewed retrospectively in terms of the system used, date LA treatment was commenced and vascular access method employed. The volumes of plasma or whole blood treated were also recorded.
Statistical analysisThe variables were investigated via visual (histograms and probability plots) and analytical methods (Kolmogorov–Smirnov and Shapiro–Wilk’s tests) for the assessment of normal distribution. Descriptive statistics were reported as means and standard deviations (SDs) for normally distributed variables and medians, minimum and maximum values for the non-normally distributed and ordinal variables.
The distribution of medians between groups was compared with the Kruskal–Wallis test. Pair to pair comparisons were performed with the Mann–Whitney U test. The distribution of ordinal and nominal variables between two and more groups was compared using the Chi-square and Fisher tests, respectively.
The statistical software package IBM SPSS Statistics for Windows, version 20.0 (IBM Corp. released 2011. Armonk, NY, USA), was used for all statistical analyses. A two-sided type-I error of 5% was used to infer statistical significance in all analyses.
ResultsA total of 27 patients, of whom 19 (70.4%) were male and 8 (29.6%), female, were included. The median age was 33 years (3–65). Eighteen patients (66.7%) were diagnosed with non-FH, 6 (22.2%), with HoFH and 3 (11.1%), with HeFH. A total of 250 apheresis procedures were performed.
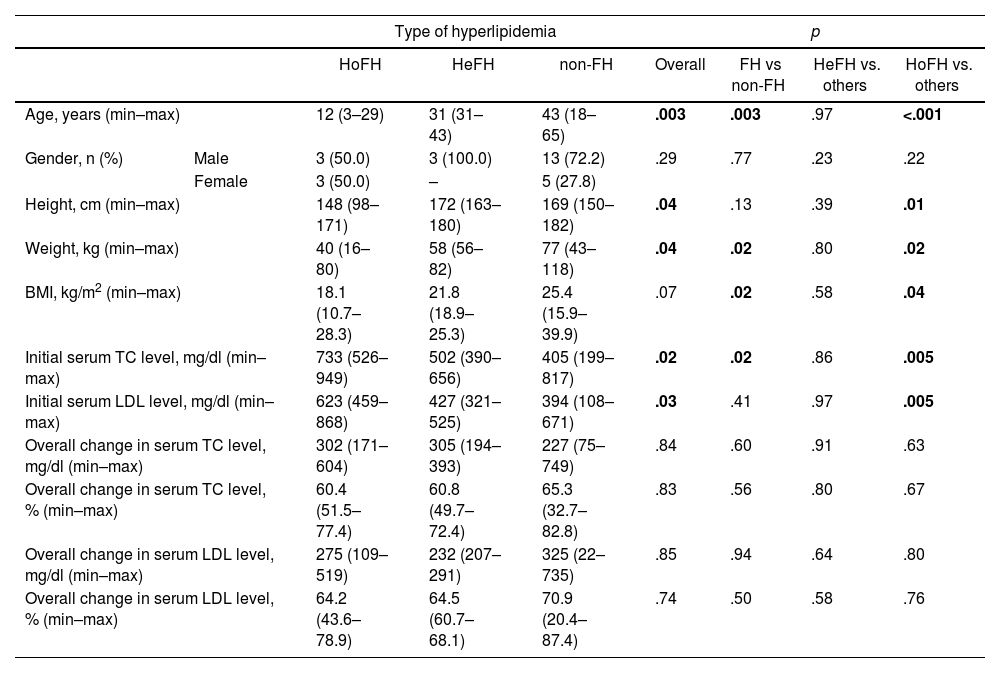
There was a significant variation in the age at which the lipoprotein apheresis procedure was initiated. LA was commenced earlier in the HoHF patients, with the median age of 12 years (3–29), than others (p < .001), as expected. The median age before starting LA for the HeFH patients was 31 years (31–43) and 43 years (18–65) for the non-FH patients. The median height, median weight and body median mass index (BMI) were lower in the HoHF patients than those in patients with HeFH and non-FH [148 cm (98–171), p = .01; 40 kg (16–80), p = .02, and; 18.1 kg/m2 (10.7–28.3), p = .04, respectively] (Table 1).
General characteristics of patients according to the type of hyperlipidemia.
| Type of hyperlipidemia | p | |||||||
|---|---|---|---|---|---|---|---|---|
| HoFH | HeFH | non-FH | Overall | FH vs non-FH | HeFH vs. others | HoFH vs. others | ||
| Age, years (min–max) | 12 (3–29) | 31 (31–43) | 43 (18–65) | .003 | .003 | .97 | <.001 | |
| Gender, n (%) | Male | 3 (50.0) | 3 (100.0) | 13 (72.2) | .29 | .77 | .23 | .22 |
| Female | 3 (50.0) | – | 5 (27.8) | |||||
| Height, cm (min–max) | 148 (98–171) | 172 (163–180) | 169 (150–182) | .04 | .13 | .39 | .01 | |
| Weight, kg (min–max) | 40 (16–80) | 58 (56–82) | 77 (43–118) | .04 | .02 | .80 | .02 | |
| BMI, kg/m2 (min–max) | 18.1 (10.7–28.3) | 21.8 (18.9–25.3) | 25.4 (15.9–39.9) | .07 | .02 | .58 | .04 | |
| Initial serum TC level, mg/dl (min–max) | 733 (526–949) | 502 (390–656) | 405 (199–817) | .02 | .02 | .86 | .005 | |
| Initial serum LDL level, mg/dl (min–max) | 623 (459–868) | 427 (321–525) | 394 (108–671) | .03 | .41 | .97 | .005 | |
| Overall change in serum TC level, mg/dl (min–max) | 302 (171–604) | 305 (194–393) | 227 (75–749) | .84 | .60 | .91 | .63 | |
| Overall change in serum TC level, % (min–max) | 60.4 (51.5–77.4) | 60.8 (49.7–72.4) | 65.3 (32.7–82.8) | .83 | .56 | .80 | .67 | |
| Overall change in serum LDL level, mg/dl (min–max) | 275 (109–519) | 232 (207–291) | 325 (22–735) | .85 | .94 | .64 | .80 | |
| Overall change in serum LDL level, % (min–max) | 64.2 (43.6–78.9) | 64.5 (60.7–68.1) | 70.9 (20.4–87.4) | .74 | .50 | .58 | .76 | |
TC: total cholesterol; LDL: low-density lipoprotein cholesterol; HeFH: heterozygous familial hypercholesterolemia; HoFH: homozygous familial hypercholesterolemia; non-FH: non-familial hypercholesterolemia.
Initial total cholesterol (TC) and LDL levels in HoHF, HeFH and non-FH patients are shown in Table 1. Initial TC and LDL levels were highest among the HoFH patients, p = .005. The median overall decreases in the serum TC levels were 302 mg/dl (171–604) (60.4%) in HoFH patients, 305 mg/dl (194–393) (60.8%) in HeFH patients and 227 mg/dl (75–749) (65.3%) in non-FH patients. The median overall decreases in serum LDL levels were 275 mg/dl (109–519) (64.2%), 232 mg/dl (207–291) (64.5%) and 325 mg/dl (22–735) (70.9%) in patients with HoFH, HeFH and non-FH, respectively. The median overall decreases in serum TC and LDL levels were similar among these patients (p > .05) (Table 1).
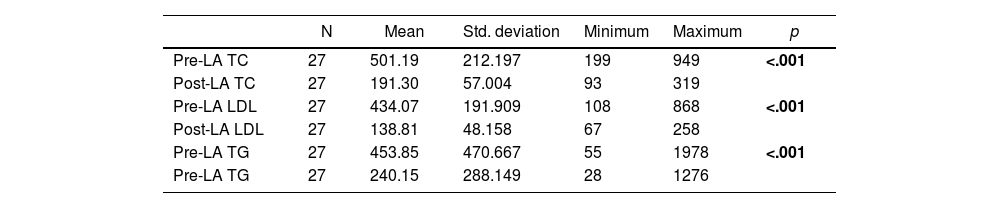
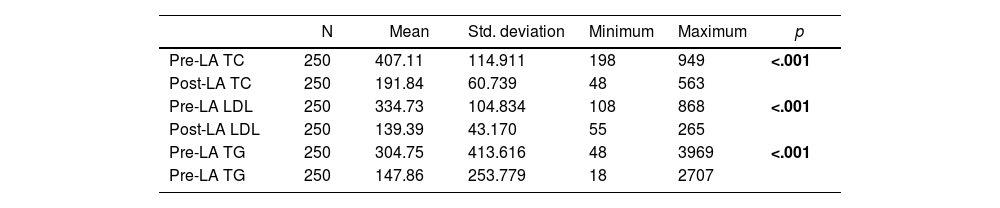
The mean pre-LA and post-LA serum TC, LDL and TG levels are shown in Table 2.1. The overall reduction in serum lipid levels, regardless of the apheresis technique used, is presented in Table 2.1. The overall reduction in serum lipid levels per session, regardless of the apheresis technique used, is shown in Table 2.2. Significant reductions in serum lipid levels, including TC, LDL and TG, were achieved (p < .001, p < .001 and p < .001, respectively) (Table 2.1). In addition, LA enabled effective reduction of serum lipid levels per session (p = <.001, p = <.001 and p = <.001, respectively) (Table 2.2).
Overall reduction in serum lipid level.
| N | Mean | Std. deviation | Minimum | Maximum | p | |
|---|---|---|---|---|---|---|
| Pre-LA TC | 27 | 501.19 | 212.197 | 199 | 949 | <.001 |
| Post-LA TC | 27 | 191.30 | 57.004 | 93 | 319 | |
| Pre-LA LDL | 27 | 434.07 | 191.909 | 108 | 868 | <.001 |
| Post-LA LDL | 27 | 138.81 | 48.158 | 67 | 258 | |
| Pre-LA TG | 27 | 453.85 | 470.667 | 55 | 1978 | <.001 |
| Pre-LA TG | 27 | 240.15 | 288.149 | 28 | 1276 |
Overall reduction in serum lipid levels per session.
| N | Mean | Std. deviation | Minimum | Maximum | p | |
|---|---|---|---|---|---|---|
| Pre-LA TC | 250 | 407.11 | 114.911 | 198 | 949 | <.001 |
| Post-LA TC | 250 | 191.84 | 60.739 | 48 | 563 | |
| Pre-LA LDL | 250 | 334.73 | 104.834 | 108 | 868 | <.001 |
| Post-LA LDL | 250 | 139.39 | 43.170 | 55 | 265 | |
| Pre-LA TG | 250 | 304.75 | 413.616 | 48 | 3969 | <.001 |
| Pre-LA TG | 250 | 147.86 | 253.779 | 18 | 2707 |
TC: total cholesterol; LDL: low-density lipoprotein cholesterol; TG: triglycerides; LA: lipoprotein apheresis.
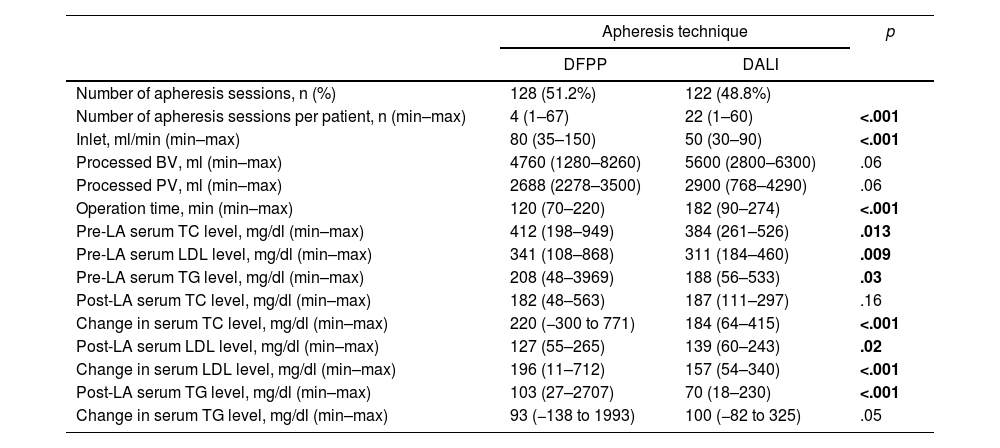
Among the LA techniques, DFPP was used in 51.2% (n = 128) and DALI, in 48.8% (n = 122), in the total number of procedures (n = 250). The numbers of apheresis sessions per patient were 4 (1–67) for DFPP and 22 (1–60) for DALI. In the DALI technique, more apheresis sessions were applied per patient (p < .001). Inlet was recorded at 80 ml/min (35–150) in the DFPP technique and 50 ml/min (30–90) in the DALI. There was a significant difference in terms of inlet between the DFPP and DALI techniques (p < .001). The processed blood volume (BV) was 4760 ml (1280−8260) in the DFPP and 5600 ml (2800−6300) in the DALI. The processed plasma volume (PV) was 2688 ml (2278−3500) in the DFPP and 2900 ml (768−4290) in the DALI procedure. The processed BV and PV were similar in the DFPP and DALI techniques, p = .06 and p = .06, respectively. The operation time was significantly shorter in the DFPP, compared to the DALI, at 120 min (70–220) vs. 182 min (90–274), p < .001, (Table 3).
The performance of different lipid apheresis techniques.
| Apheresis technique | p | ||
|---|---|---|---|
| DFPP | DALI | ||
| Number of apheresis sessions, n (%) | 128 (51.2%) | 122 (48.8%) | |
| Number of apheresis sessions per patient, n (min–max) | 4 (1–67) | 22 (1–60) | <.001 |
| Inlet, ml/min (min–max) | 80 (35–150) | 50 (30–90) | <.001 |
| Processed BV, ml (min–max) | 4760 (1280–8260) | 5600 (2800–6300) | .06 |
| Processed PV, ml (min–max) | 2688 (2278–3500) | 2900 (768–4290) | .06 |
| Operation time, min (min–max) | 120 (70–220) | 182 (90–274) | <.001 |
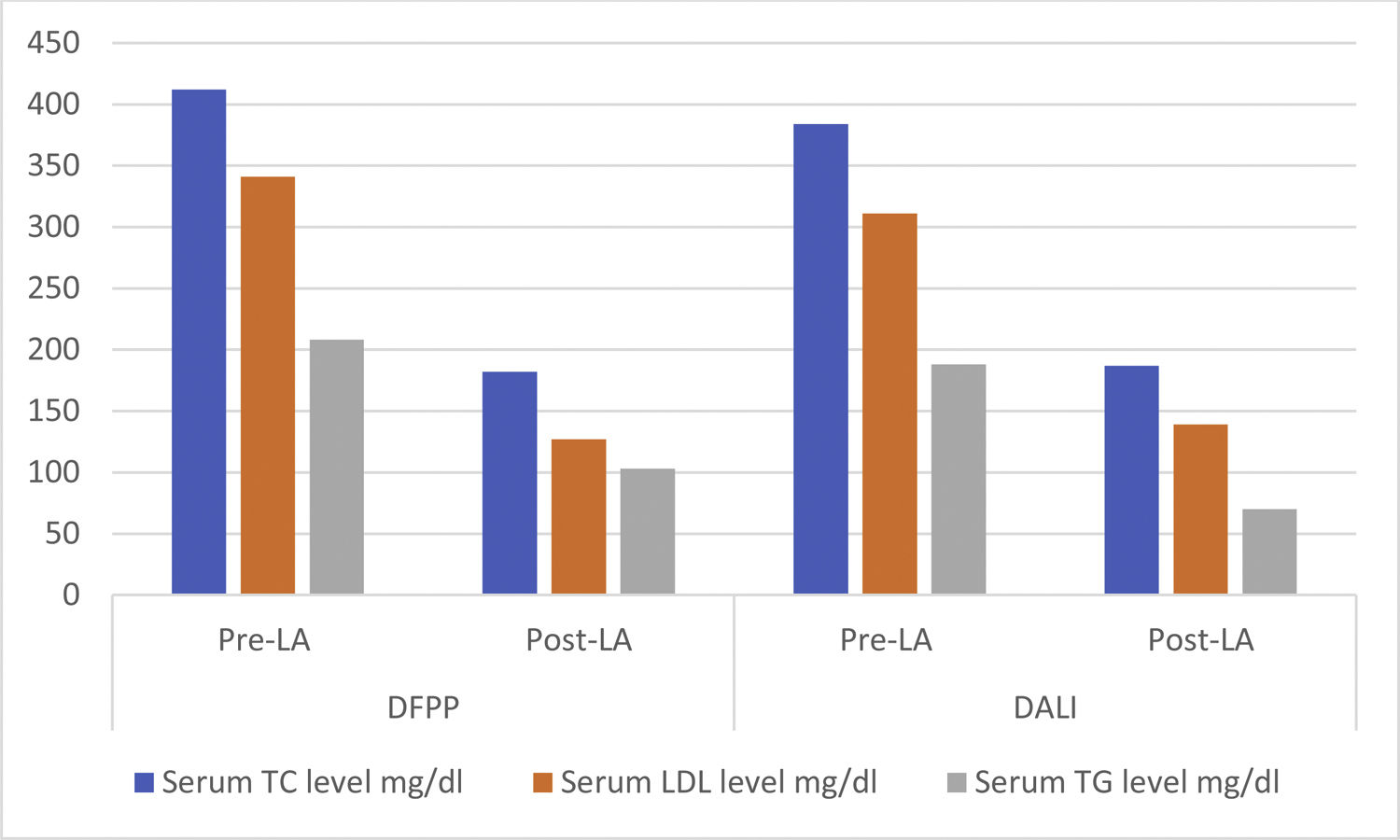
| Pre-LA serum TC level, mg/dl (min–max) | 412 (198–949) | 384 (261–526) | .013 |
| Pre-LA serum LDL level, mg/dl (min–max) | 341 (108–868) | 311 (184–460) | .009 |
| Pre-LA serum TG level, mg/dl (min–max) | 208 (48–3969) | 188 (56–533) | .03 |
| Post-LA serum TC level, mg/dl (min–max) | 182 (48–563) | 187 (111–297) | .16 |
| Change in serum TC level, mg/dl (min–max) | 220 (−300 to 771) | 184 (64–415) | <.001 |
| Post-LA serum LDL level, mg/dl (min–max) | 127 (55–265) | 139 (60–243) | .02 |
| Change in serum LDL level, mg/dl (min–max) | 196 (11–712) | 157 (54–340) | <.001 |
| Post-LA serum TG level, mg/dl (min–max) | 103 (27–2707) | 70 (18–230) | <.001 |
| Change in serum TG level, mg/dl (min–max) | 93 (−138 to 1993) | 100 (−82 to 325) | .05 |
DFPP: double filtration plasmapheresis; DALI: direct adsorption of lipoproteins; BV: blood volume; PV: plasma volume; LA: lipoprotein apheresis; TC: total cholesterol; LDL: low-density lipoprotein cholesterol; TG: triglycerides.
Pre-LA and post-LA serum TC, LDL and TG levels, in the DFPP and DALI techniques are presented in Table 3. The reduction in the serum TC level was significantly higher in the DFPP technique, compared to the DALI; 220 mg/dl (−300 to 771) vs. 184 mg/dl (64–415), p < .001. The decrease in the serum LDL level was 196 mg/dl (11–712) in the DFPP and 157 mg/dl (54–340) in the DALI, p < .001. The change in the serum TG level did not reach statistical significance between the DFPP and DALI procedures; 93 mg/dl (−138 to 1993) vs. 100 mg/dl (−82 to 325), p = .05, respectively, (Table 3, Figure 1).
Two hundred and twenty-six (90.4%) procedures were performed via peripheral veins, 17 (6.8%) were performed through a transient central venous access and 7 (2.8%) were performed via an arterio-venous (AV) fistula. No major procedure-related complications were observed. A vascular access problem was reported in a patient who underwent LA via peripheral veins.
DiscussionHigh LDL cholesterol levels may lead to premature coronary heart disease, even in the absence of any other risk factors. Lipid-lowering medications are not always successful in reducing increased LDL levels. LDL-apheresis is often used for the treatment of severe types of FH patients who are resistant to lipid-lowering pharmacotherapy. Healthcare providers are recommended to consider LA for decreasing serum Lp(a) and LDL levels, which is associated with a reduced cardiovascular risk.17,18
LA techniques are divided into two groups, the selective and non-selective methods. The DALI is the first selective low LDL apheresis technology, by which LDL and Lp(a) can be selectively removed from whole blood without plasma separation.4 At our center, the DFPP (51.2%) and DALI (48.8%) techniques were used, both of which are selective methods. The number of apheresis sessions per patient was higher in the DALI group, (p < .001). In addition, the operation time was significantly lower in the DFPP procedure (p < .001) and it seems that fewer apheresis sessions per patient were needed in this technique.
Previous studies suggested that the effectiveness of all selective methods, including the DALI and DFPP, in lowering LDL (approximately 55%–70%, after a single treatment) and Lp(a) mass (50%–60%) is roughly similar.19–21 In our study, the reduction in the serum TC level was significantly higher in the DFPP technique, compared to the DALI, p < .001. Despite a higher initial level of LDL observed in the DFPP group, a lower serum LDL level was achieved after LA with the DFPP, compared to the DALI; 127 mg/dl (55–265) vs. 139 mg/dl (60–243), p = .02. A significant overall decrease in the serum lipid levels, including TC, LDL and TG, was achieved, regardless of the apheresis technique used (p < .001, p < .001 and p < .001, respectively). Our results indicate that LA is highly efficient in lowering serum TC, LDL and TG levels, regardless of the method used. However, the DFPP method seems to be more advantageous than the DALI method, since it provided a higher decrease in total cholesterol and LDL, in a shorter operation time.
Moriarty et al. reported a 94% reduction in major adverse cardiovascular events over a mean treatment period of 48 months with LA therapy, as a result of a reduction of 64% and 63% in LDL and Lp(a), respectively.22 Previous studies reported a mean range of 57–75% in LDL reduction after LA among individuals with HoFH and 58–63% among individuals with HeFH.23 LA also decreased inflammatory markers and blood viscosity.24 In HoFH patients, we have also achieved an overall decrease of 60.4% and 64.2% for serum TC and LDL levels, respectively. The reductions in serum TC and LDL levels were similar among individuals with HoFH, HeFH and non-FH. As previously mentioned, lipoprotein apheresis is a highly effective treatment for lowering serum LDL and total cholesterol levels. The success of the procedure can be increased by choosing the appropriate technique.
Schmöcker et al. reported that total triglyceride levels were reduced more potently by membrane filtration apheresis, compared to heparin precipitation and direct absorption.25 In our study, the pre-LA serum TG level was higher in the DFPP group, compared to the DALI. Although the change in the TG level did not reach statistical significance (p = .05), a lower serum TG level was achieved in the DALI procedure after LA. We suggested that the DALI technique was more effective in decreasing the serum TG.
A study that evaluates health and quality of life (QoL) in apheresis patients showed that many of the patients undergoing apheresis treatment have poor health and QoL at the start of therapy. Unexpectedly, the level of QoL was high, especially for patients undergoing LA, compared to other apheresis procedures, despite the continuous long-term treatment they were bound to.26 The HoFH patients undergoing LDL-apheresis mentioned that LA is time-consuming, uncomfortable and difficult to cope with.27 However, Bosch et al. reported that long-term therapy with the DALI was safe, effective and selective, as it was possible to reduce the LDL-C and Lp(a) by >60% per session in an approximately 100-min treatment. A low incidence of adverse events was observed.4 According to the 5-year results of the German Lipoprotein Apheresis Registry, LA treatments were maximally tolerated with lipid-lowering medication and found to be safe, with a low rate of side effects.10 In our study, the median operation time was 120 min (70–220) in the DFPP and 182 min (90–274) in the DALI method. No major adverse event occurred.
The Austrian experience in lipoprotein apheresis showed that regular LA at weekly intervals was significantly effective in reducing major cardiovascular events and was associated with prolonged event-free survival for patients at risk.28 The annual cost was estimated at US$ 66,374 in France and Germany per patient for a weekly treatment (US$ 33,187 for a biweekly treatment) with LA in 2015. Wang et al. claimed that the frequency of the guideline-recommended treatment resulted in substantial annual costs, which is a barrier in the optimal treatment of FH.23 In recent days, novel lipid-lowering agents have the potential to improve the performance of LA, but more evidence is needed.19 Considering that patients with familial hypercholesterolemia should receive treatment for almost their whole lifetimes, regular LA treatment may be the rationale for increasing the therapeutic effect of novel lipid-lowering drugs and reducing the cost.
The main limitations of our study were the retrospective design and the absence of data on the cardiovascular outcome. However, it provides real-life experience in lipoprotein apheresis and comparison of two LA methods, namely the DFPP and DALI.
ConclusionLipoprotein apheresis is a well-established extracorporeal technique for lowering the serum lipid levels, such as LDL, TC and TG, regardless of the apheresis technique used. Our data revealed that the DFPP technique was more effective in the reduction of the serum TC and LDL levels in a shorter operation time, compared to the DALI. In addition, the DALI method was more efficient in decreasing the serum TG level than the DFPP. The success of the LA and QoL of patients may be improved by choosing the appropriate LA technique, while the cost and duration of procedures may also be reduced. In addition, we suggest that LA is a highly efficient treatment in patients with HoFH and HeFH, particularly at high risk for atherosclerotic heart disease. There is an unmet need for effective guidelines on how and how often LA should be combined with novel lipid-lowering agents in individuals at high risk for cardiovascular disease.
Conflicts of interestThe authors declare no conflicts of interest.