Pre-apheresis peripheral blood CD34+ cell count (PBCD34+) is the most important predictor of good cell mobilization before hematopoietic stem cell transplantation, albeit flow cytometry is not always immediately available. Identification of surrogate markers can be useful. The CD34+ cells proliferate after mobilization, resulting in elevated lactate dehydrogenase (LDH) activity and correlating with the PBCD34+ count.
ObjectiveTo determine the LDH cut-off value at which adequate CD34+ cell mobilization is achieved and its diagnostic yield.
Materials and methodsA total of 103 patients who received an autologous stem cell transplantation (ASCT) between January 2015 and January 2020 were included. Demographic and laboratory characteristics were obtained, including complete blood count, pre-apheresis PBCD34+ and LDH levels. Receiver operating characteristic (ROC) curves were performed to identify the optimal serum LDH activity cut-off points for ≥ 2 and ≥ 4 × 106 cells/kg post-mobilization CD34+ count and their diagnostic yield.
ResultsA post-mobilization serum LDH cut-off value of 462 U/L yielded a sensitivity (Se) = 86.8% (positive predictive value [PPV] = 72.7%), a pre- and post-mobilization serum LDH difference cut-off value of 387 U/L, an Se = 45.7% (PPV = 97%) and an LDH ratio of 2.46, with an Se = 47.1% (PPV = 97%) for an optimal mobilization count (CD34+ ≥ 4 × 106).
ConclusionThe LDH measurement represents a fast and affordable way to predict PBCD34+ mobilization in cases where flow cytometry is not immediately available. According to the LDH diagnostic yield, it could be used as a surrogate marker in transplant centers, supporting the CD34+ count, which remains the gold standard.
Autologous stem cell transplantation (ASCT) has been widely used as a therapeutic procedure for the treatment of hematologic disorders, such as leukemia, lymphoma and multiple myeloma (MM), among others.1–3
Peripheral blood stem cells (PBSCs) are collected by apheresis after mobilizing CD34+ cells by a granulocyte-colony-stimulating factor (G-CSF), with or without chemotherapy. In poor mobilizers, plerixafor can be added to increase mobilization.4–6 A CD34+ cell count of at least 2 × 106 cells/kg is commonly required for autologous stem cell grafts to guarantee hematopoietic recovery after myeloablative chemotherapy.7,8 In some patients, two or more aphereses are needed to obtain the ideal number of CD34+ cells.9,10 Factors, such as age, obesity, prior therapies and comorbidities, can influence the ability to collect PBSCs, even with the same mobilization regimen.11–19
Pre-apheresis peripheral blood CD34 determination (PBCD34) is a surrogate measure for hematopoietic stem cells and is the most accurate predictor of a stem cell yield.5 However, PBCD34 determination requires a flow cytometer and a trained technician; thus, its absence may delay the apheresis process.10,20 Surrogate markers, such as leukocyte, platelet count and serum lactate dehydrogenase, which predict good mobilization, have been proposed as easy and rapid alternatives to PBCD34.9,21 Predicting poor mobilization may allow early intervention and prevent the expense and potential complications associated with a second apheresis.
Lactate dehydrogenase (LDH) is a predominantly cytoplasmatic enzyme that plays a role in the anaerobic metabolic pathway. Increased LDH levels in plasma are mainly related to lysis and tissue turnover.22,23. After G-CSF mobilization, circulating CD34+ cells proliferate, resulting in elevated LDH activity.24 The LDH has emerged as a candidate surrogate marker for adequate stem cell mobilization, as previous studies have reported a positive correlation between serum LDH and circulating CD34+ cells.25,26 An LDH cut-off value as a surrogate marker for CD34+ cell mobilization has been reported; it was found that patients with a difference > 300 U/L of LDH levels in pre- and post-mobilization had a good mobilization, with a strong correlation (rho = 0.55, p = 0.001), compared to the PBCD34+ count; however, this was an arbitrary value and its diagnostic yields was not determined.27 The aim of this retrospective study was to determine the LDH cut-off value at which adequate CD34+ cell mobilization is achieved and its diagnostic yield for ≥ 2 and ≥ 4 × 106 cells/kg CD34+ cell counts.
Materials and methodsPatientsThis retrospective study included, between January 2015 and January 2020, all consecutive patients older than 11 years who underwent ASCT indicated as first-line therapy at our hematological referral center, after reaching at least a partial response to induction therapy in the case of hematological malignancies, complete remission in neurological and rheumatological conditions and during the “honeymoon phase” in type 1 diabetes, according to corresponding clinical guidelines and consensus for each disease. All patients who met the following criteria before transplant were included: an Eastern Cooperative Oncology Group (ECOG) performance status score < 2, normal liver function tests and a complete blood cell count compatible with an adequate marrow function (white blood cell [WBC] count ≥ 3,000/μL, absolute neutrophil count ≥ 1,500/μL and platelet count ≥ 100,000/μL). Patients with serum creatinine ≥ 2.2 mg/dL, a positive immunological pregnancy test, a lack of LDH determination, or any clinical condition considered high-risk for HSCT were excluded.
Mobilization regimen and cell collectionStem cell harvests were performed after the use of biosimilar standard G-CSF28 at a dose of 10 mg/kg/day subcutaneously for 4 days from days 1 to 4, with or without plerixafor at a single dose of 0.12 mg/kg administered subcutaneously, 11 hours before leukapheresis.29 Additional plerixafor was administered to some patients taking G-CSF due to ongoing clinical trials at our center and these were also included in the retrospective analysis.
The apheresis procedure was performed on day 5 and allowed a minimum peripheral blood CD34+ cell count of ≥ 8 cells/μL, according to local guidelines, to obtain ≥ 2 × 106 CD34+ cells/kg in a single procedure utilizing a Spectra Optia® apheresis system (Terumo BCT) or an Amicus® separator system (Fresenius Kabi). Due to local protocols, a maximum of two apheresis procedures were performed to obtain a successful cell harvest, if required. The CD34+ cell counts were obtained in peripheral blood samples by flow cytometry in a Beckton Dickinson Accuri C6 flow cytometer (BD Biosciences) using a BD Stem Cell Enumeration kit.
The LDH was measured just before mobilization and immediately prior to the apheresis procedure on day 5. The LDH was determined by spectrophotometry using the LDHI2 kit (Roche Diagnostics, Switzerland) and was measured by a Cobe spectrophotometer (Roche Diagnostics, Switzerland). The normal reference values previously determined in our laboratory were 135 - 214 U/L for women and 135 - 225 U/L for men.
The CD34+ PBSCs pre-counts were also assessed before the apheresis procedure on day 5.
All patient data were obtained from their clinical files and electronic laboratory databases. We considered a good mobilization ≥ 2 × 106 CD34+ cells/kg and an optimal mobilization ≥ 4 × 106 CD34+ cells/kg.
Conditioning regimen and autograft infusionThe conditioning regimen was administered to all individuals as outpatients. In short, high doses of melphalan were used in multiple myeloma patients; for lymphomas, the regimen was chosen according to the subclassification of this malignancy. Autologous CD34+ stem cells were not frozen, but were stored at 4° C in a conventional blood bank refrigerator and were infused on day 0 through a central catheter or peripheral vein. Starting on day 0, all patients received oral prophylaxis with levofloxacin, acyclovir and itraconazole or voriconazole.
Statistical analysisPatient characteristics and clinical features were described as frequencies and percentages. Numerical variables were reported in median (interquartile range) after non-parametric distribution identification with the Kolmogorov-Smirnov test. The LDH difference was calculated as the difference between the post- and pre-mobilization serum LDH values, while the LDH ratio was calculated as the coefficient of the post- and pre-mobilization serum LDH values. Serum LDH parameters and CD34+ cell counts between mobilization regimens were compared with the Mann-Whitney U test. In addition, we assessed the degree of association between the serum LDH activity and post-mobilization CD34+ count with the Spearman correlation coefficient (rho). Receiver Operating Characteristic (ROC) curves were performed to identify optimal serum LDH activity cut-off points and diagnostic yields (sensitivity, specificity, PPV [positive predictive value] and NPV [negative predictive value]) of selected values, depending on the highest Youden Index for each curve for good mobilization (≥ 2 × 106 CD34+) and optimal mobilization (≥ 4 × 106 CD34+) post-mobilization CD34+ count. Statistical significance was set at a p-value < 0.05 and the 95% confidence interval (95% CI) was calculated.
ResultsA total of 103 apheresis procedures for autologous transplantation were performed from 2015 to 2020 at our center. The median age was 49 years (range: 11-72), 54 were women and 49 were men. The patient primary diagnoses and ASCT indications were MM (n = 49, 47.6%), non-Hodgkin's lymphoma (n = 26, 25.2%), followed by type 1 diabetes (n = 14, 13.6%) and Hodgkin's lymphoma (n = 7, 6.8%), among others (multiple sclerosis, juvenile idiopathic arthritis, primary amyloidosis, acute lymphoblastic leukemia, acute myeloid leukemia and POEMS syndrome) (Table 1). Sixty-eight apheresis procedures (67%) were performed after mobilization with filgrastim and, in thirty-five (33%), the mobilization regimen consisted of filgrastim plus plerixafor. A median of 1 (1 - 2) apheresis was performed to reach a successful cell harvest.
Correlation between LDH activity parameters and CD34+ cell counts.
rho = Spearmen correlation coefficent.
The median pre-mobilization serum LDH level was 296 (255 - 359) U/L; after mobilization, it was 584 (478-739) U/L. The post-mobilization serum LDH values among patients who received filgrastim, with or without plerixafor, were not significantly different (620.5 [486.7 - 777.2] U/L vs. 579 [439 - 703] U/L, p = 0.295), nor were the post- and pre-mobilization serum LDH differences (321 [175.5 - 484.7] U/L vs. 256 [167 - 428] U/L, p = 0.394) and the LDH ratio (2.02 [1.51-2.57] vs. 1.98 [1.48 - 2.83], p = 0.795) between both groups. The overall median post-mobilization CD34+ count was 5 (2.74-8) cells/μL. The post-mobilization CD34+ count was higher in the group of patients who received plerixafor in addition to G-CSF than in those with G-CSF alone (5.4 [4 - 12.1] μ/L vs. 4 [2 - 6.5] μ/L, p = 0.020).
Correlations and ROC analysesPositive moderate significant correlations were observed between the post-mobilization serum LDH, LDH difference and LDH ratio and total CD34+ counts, while a low correlation between the latter and pre-mobilization serum LDH values was reached (Table 1).
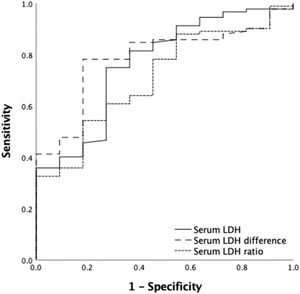
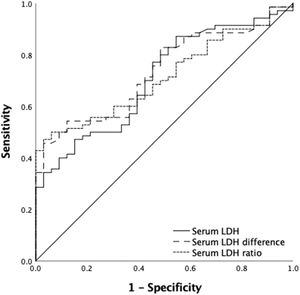
A ROC curve analysis was performed to identify the serum LDH activity cut-off point associated with successful CD34+ mobilization. For a good mobilization (CD34+ count ≥ 2 × 106 cells/kg), a post-mobilization serum LDH cut-off value of 493 U/L yielded a sensitivity of 75.9% and a specificity of 72.7% (PPV = 95.8%; NPV = 25.8%), whereas a post- and pre-mobilization LDH difference cut-off point of 179 U/L yielded a sensitivity of 78.3% and a specificity of 81.8% (PPV = 97.2%; NPV = 31%) and a LDH ratio cut-off of 2 yielded a sensitivity and specificity of 54.3% and 81.8%, respectively (PPV = 96.1%; NPV: 17.6%) (Table 2) (Figure 1).
Comparison of ROC curve analysis values between ≥ 2 × 106 CD34+ cut-off value, LDH and WBC.
| Parameters | Cut-off value | Se (%) | Sp (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Post-mobilization LDH, U/L | 493 | 75.9 | 72.7 | 95.8 | 25.8 |
| LDH difference*, U/L | 179 | 78.3 | 81.8 | 97.3 | 31 |
| LDH ratioa | 2 | 54.3 | 81.8 | 96.1 | 17.6 |
| Leucocytes/ µL | 36.5 | 52.9 | 72.7 | 93.8 | 93.8 |
| Monocytes/ µL | 3.5 | 79.3 | 81.8 | 97.2 | 97.2 |
Se: sensitivity; Sp: specificity; PPV: positive predictive value; NPV: negative predictive value; LDH: lactate dehydrogenase; WBC: white blood cells.
For an optimal mobilization (CD34+ count ≥ 4 × 106 cells/kg), we also identified a post-mobilization serum LDH cut-off point of 462 U/L, which gave a sensitivity of 86.8 % and a specificity of 43.3% (PPV = 77.2%; NPV = 62.5%) and a post- and pre-mobilization serum LDH difference cut-off of 387 U/L, which reached a sensitivity of 45.7% and a specificity of 97% (PPV = 97%; NPV = 45.7%) and an LDH ratio cut-off of 2.46 yielded a sensitivity and specificity of 47.1% and 97 %, respectively (PPV = 97%; NPV: 46.3%) (Table 3) (Figure 2).
Comparison of ROC curve analysis values between ≥ 4 CD34+ cut-off value, LDH and WBC.
| Parameters | Cut-off value | Se (%) | Sp (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Post-mobilization LDH, U/L | 462 | 86.8 | 43.3 | 72.7 | 62.5 |
| LDH difference*, U/L | 387 | 45.7 | 97 | 97 | 45.7 |
| LDH ratioa | 2.46 | 47.1 | 97 | 97 | 46.3 |
| Leucocytes/ µL | 38.5 | 52.9 | 70 | 80 | 39.6 |
| Monocytes/ µL | 3.5 | 45.6 | 96.7 | 96.9 | 45.7 |
Se: sensitivity; Sp: specificity; PPV: positive predictive value; NPV: negative predictive value; LDH: lactate dehydrogenase; WBC: white blood cells.
We performed a ROC sub-analysis for identifying the post-mobilization serum LDH, pre- and post-mobilization serum LDH differences, and pre- and post-mobilization serum LDH cut-off points for good and optimal mobilization of CD34+ cells, adjusted by the use of plerixafor in addition to G-CSF as mobilization regimens, which are summarized in Table 4. All chosen cut-offs reached a > 80% PPV for good and optimal CD34+ cell harvest after mobilization with G-CSF, with and without plerixafor, excluding for the selected best cut-off point for post-mobilization LDH for optimal CD34+ cell mobilization in patients who received plerixafor in addition to G-CSF.
Comparison of ROC curve analysis values between ≥ 4 CD34+ cut-off value, LDH and WBC.
| CD34+ cut-off | Plerixafor addition | Parameters | Cut-off value | AUC (95% CI) | Se (%) | Sp (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| ≥ 2 × 106 cells/kg | Yes | Post-mobilization LDH, U/L | 446.5 | 0.874 (0.695 - 1) | 86.2 | 83.3 | 96 | 50 |
| LDH difference*, U/L | 182 | 0.759 (0.565 - 0.952) | 75.9 | 83.3 | 95.6 | 41.7 | ||
| LDH ratioa | 1.9 | 0.569 (0.350 - 0.788) | 58.6 | 66.7 | 89.5 | 13.3 | ||
| No | Post-mobilization LDH, U/L | 498 | 0.67 (0.454 - 886) | 76.2 | 60 | 96 | 35.7 | |
| LDH difference*, U/L | 178 | 0.803 (0.641 - 0.965) | 79.4 | 80 | 98 | 23.5 | ||
| LDH ratioª | 1.4 | 0.844 (0.693 - 0.996) | 88.9 | 80 | 98.2 | 16.7 | ||
| ≥ 4 × 106 cells/kg | Yes | Post-mobilization LDH, U/L | 561 | 0.745 (0.583 - 0.907) | 72.2 | 64.7 | 68.4 | 68.7 |
| LDH difference*, U/L | 399 | 0.747 (0.582 - 0.912) | 50 | 94.1 | 90 | 64 | ||
| LDH ratioa | 2.58 | 0.706 (0.526 - 0.885) | 44.4 | 100 | 88.9 | 61.5 | ||
| No | Post-mobilization LDH, U/L | 498 | 0.691 (0.554 - 0.829) | 80.8 | 50 | 84 | 44.4 | |
| LDH difference*, U/L | 387 | 0.725 (0.599 - 0.852) | 44.2 | 100 | 100 | 35.5 | ||
| LDH ratioa | 2.38 | 0.736 (0.612 - 0.859) | 48.1 | 100 | 100 | 65.2 |
AUC (95% CI): area under the curve (95% confidence interval); Se: sensitivity; Sp: specificity; PPV: positive predictive value; NPV: negative predictive value; LDH: lactate dehydrogenase; WBC: white blood cells.
Fewer than the ideal number of transplants are performed in low- and middle-income countries.30 However, in recent years, efforts have been made to increase these procedures due to their therapeutic value. The existence of a fast, effective and inexpensive surrogate marker for predicting an adequate PBCD34+ can help increase the number of safer transplants in institutions and countries with scarce resources. In our study, serum LDH had a positive correlation with circulating CD34+ levels after mobilization for auto-HSCT. We found a moderate correlation between the post-mobilization LDH and the difference between pre- and post-mobilization LDH with the CD34+ count (rho = 0.485, p < 0.001; rho = 0.462, p < 0.001). The best sensitivity value (86.8%) was provided by a post-mobilization serum LDH value of 462 U/L with a PPV of 72.7%; therefore, it could be used as a screening marker for optimal CD34+ mobilization. The LDH difference and LDH ratio could also be appropriate predictors for good CD34+ (≥ 2 × 106) and optimal CD34+ (≥ 4 × 106) counts with high VPN values.
Our results are consistent with results from previous studies, which have reported a significant correlation between LDH levels and mobilization (r = 0.54, p < 0.001) (r = 0.55, p < 0.001).25,27 Egan et al. compared the correlation between serum LDH and PBCD34+ on different days and found a very strong (r = 0.54, p < 0.001) correlation overall, with a weakened relationship by day two. Donmez et al. reported a mild correlation between post-mobilization serum LDH activity and the PBCD34 count (r = 0.43, p = 0.007). Moreover, they obtained a strong correlation between the pre- and post-mobilization serum LDH activity difference and the PBCD34+ count (r = 0.55, p = 0.001). Furthermore, it is important to know that LDH determination is cheaper and, thus, affordable in low-income settings, as well as faster. At our hospital, the cost of an LDH analysis is 15 to 20 USD, while CD34+ flow cytometry costs 120 to 130 USD. On the other hand, the processing time for an LDH sample is around 60 minutes, while for the CD34+, approximately 3 hours are needed. Flow cytometry is not widely available at most centers and the turnaround time can limit its utility.
To our knowledge, the current study provides the first report assessing the sensitivity, specificity, PPV and NPV for LDH levels in predicting CD34+ cell mobilization in AHSCT patients. Moreover, our findings show that the highest specificity (97%) was obtained with an LDH cut-off level of 387 U/L, with a PPV and NPV of 97 and 45.7%, respectively. These results support the hypothesis that the serum LDH level can be an acceptable surrogate marker in case of flow cytometry unavailability; however, it should not yet be considered as a standard replacement of CD34+ determination, but as an affordable and faster predictor of the CD34+ yield that can aid in the decision-making, mostly in low-resource environments, such as ours.
A limitation of this study was its retrospective nature, its relatively modest sample size and number of patients included with malignancies in whom the LDH value could have been intrinsically elevated. Further studies with larger sample sizes representing each diagnostic category are required to confirm our findings.
ConclusionIn conclusion, LDH measurements could represent a faster and more affordable manner in which to predict good mobilization in cases when the PBCD34+ count by flow cytometry is not immediately available. An LDH cut-off value of 462 U/L provided the best marker for optimal mobilization of CD34+ cells and could be an alternative parameter used in transplant centers from low-middle income countries lacking, for any reason, a rapid flow cytometry result. However, it is important to note that the CD34+ cell count remains the gold standard in this setting.
Authors statementPerla R. Colunga-Pedraza; Mariela Irabien-Zuñiga: Conception and design of the study, drafting the article
Carlos Saúl Rodriguez Roque: Acquisition of data, Conception and design of the study, drafting the article
Carlos de la Cruz de la Cruz: Analysis and interpretation of data
Andres Gómez de León: Conception and design of the study, drafting the article
Paola Santana-Hernández: Conception and design of the study
José Carlos Jaime-Pérez: Conception and design of the study
Consuelo Mancías-Guerra: Analysis and interpretation of data
David Gómez-Almaguer: Revising the article, acquisition of data
Data availability statementThe data that support the findings of this study are available on request from the corresponding author, DGA.
Ethics approvalThe protocol was revised and approved by the Institutional Review Board (IRB) on human subject research of the Hospital Universitario “Dr. Jose Eleuterio Gonzalez” Ethics Committee.
No writing consent was required because patient's data were collected retrospectively from the study records and were used anonymously.
We thank Sergio Lozano-Rodríguez, MD for critically reading our manuscript and suggesting substantial improvements.