The Klotho protein, whose gene has predominant renal expression, acts in the control of serum phosphorus and 1,25-dihydroxyvitamin D3 and regulates the function of ion channels. It also participates in the mechanism of protection against oxidative stress and acts on the vascular endothelium by inducing the production of nitric oxide. Mutations that reflect defects in the Klotho gene expression may be implicated in the onset of osteonecrosis, priapism, and leg ulcers in patients with sickle cell disease, as a result of oxidative stress and endothelial impairment, important factors in the development and severity of this disease. Previous reports regarding the association of Klotho single nucleotide polymorphisms with sickle cell disease subphenotypes have found that these polymorphisms are important to identify genetic markers of risk in these individuals and allow early and more effective therapeutic intervention.
Klotho is a gene that consists of five exons and is located on chromosome 13q12 in humans. Its expression occurs predominantly in the kidney distal convoluted tubules and the choroid plexus of the brain. Endocrine organs (pituitary gland, parathyroid gland, pancreas, ovary, testis and placenta), the heart and pancreatic β cells also express Klotho.1–7
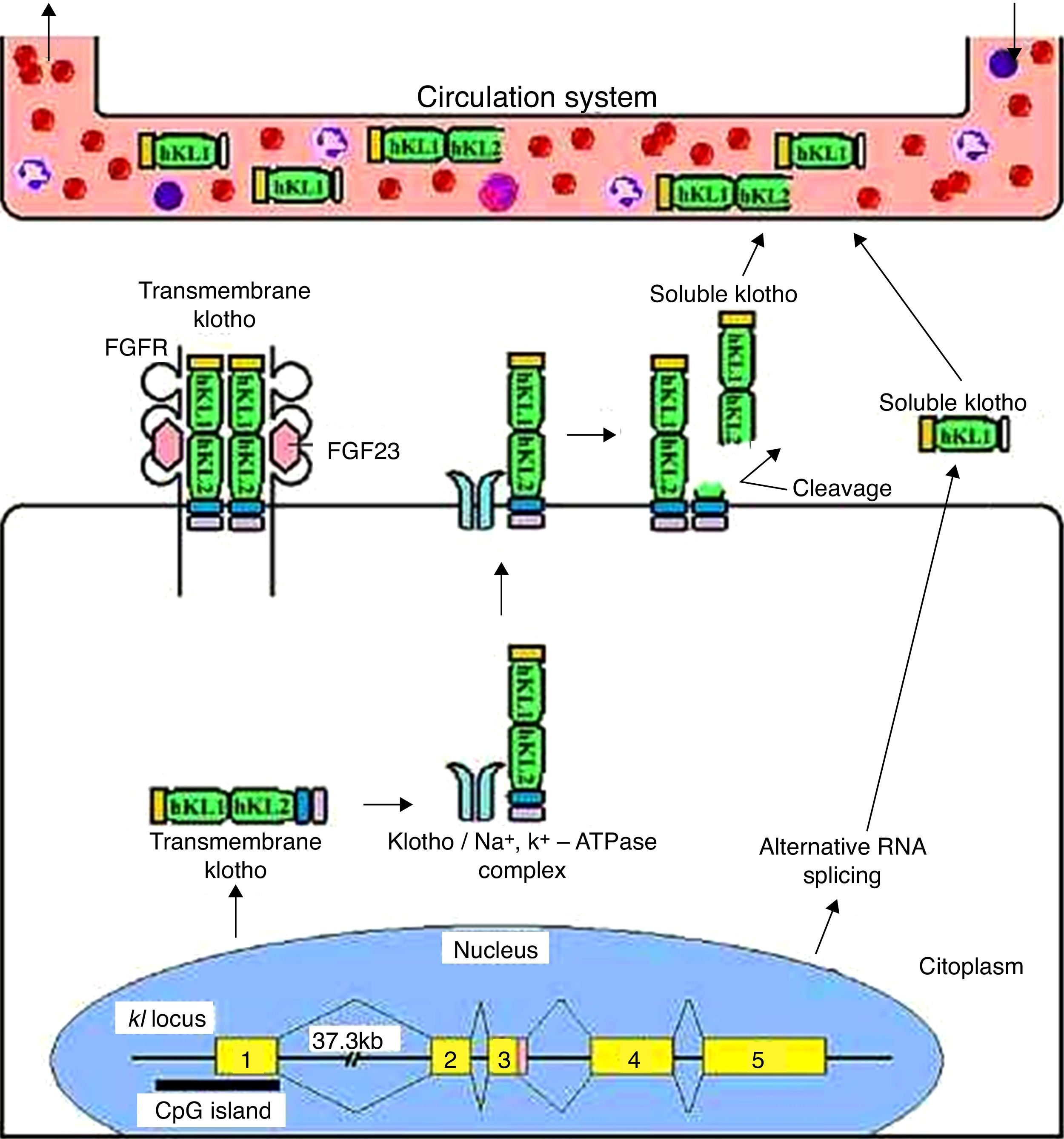
Klotho generates two transcripts, a transmembrane protein and a secreted protein, resulting from alternative splicing to the third exon. Additionally, the transmembrane protein can be cleaved by α- and β-secretases to generate a secreted protein, which is, two-times longer than the alternatively spliced transcript.4,6,8–11 So, the effects of the Klotho protein extend beyond the tissues that express the gene because of its humoral factor function. Klotho reaches the systemic circulation by secreted fractions, and is released into the extracellular space and subsequently into circulation; it is found in blood, urine and cerebrospinal fluid (Figure 1).11–13
Transmembrane and secreted Klotho protein processing. The transmembrane Klotho protein acts as a co-receptor for fibroblast growth factor 23 signaling. This transmembrane protein forms complexes with Na+/K+-ATPase channels and is recruited to the cell surface. Once on the surface, the Klotho protein is cleaved by secretases forming a soluble form of Klotho. This form enters the circulatory system similar to the form produced by alternative splicing, and acts on other organs as a humoral factor.
Multiple aging phenotypes result from defects in gene expression of Klotho,11 such as growth retardation, hyperphosphatemia, moderate hypercalcemia, vascular and soft tissue calcification, and high levels of 1,25-dihydroxyvitamin D3 (1.25(OH)2D3) and fibroblast growth factor 23 (FGF23). These defects in animals result in a shortened life expectancy when compared to wild-type phenotypes. Additionally, the kl−/− animal exhibits hypokinesia, gait changes, and atrophy of the genitals, skin and thymus.3,14Klotho single nucleotide polymorphisms (SNPs) have been associated with subphenotypes of sickle cell disease (SCA),15–19 a monogenic autosomal recessive disorder, characterized by a β-globin gene (HBB) mutation which results in the formation of the S variant hemoglobin (Hb S).
Given the possible role of Klotho as one of the genes responsible for the phenotypic variability between patients with sickle cell disease (SCD), the aim of this paper is to review the various features of the Klotho protein, as well as discuss current literature about the association between Klotho polymorphisms and SCD subphenotypes.
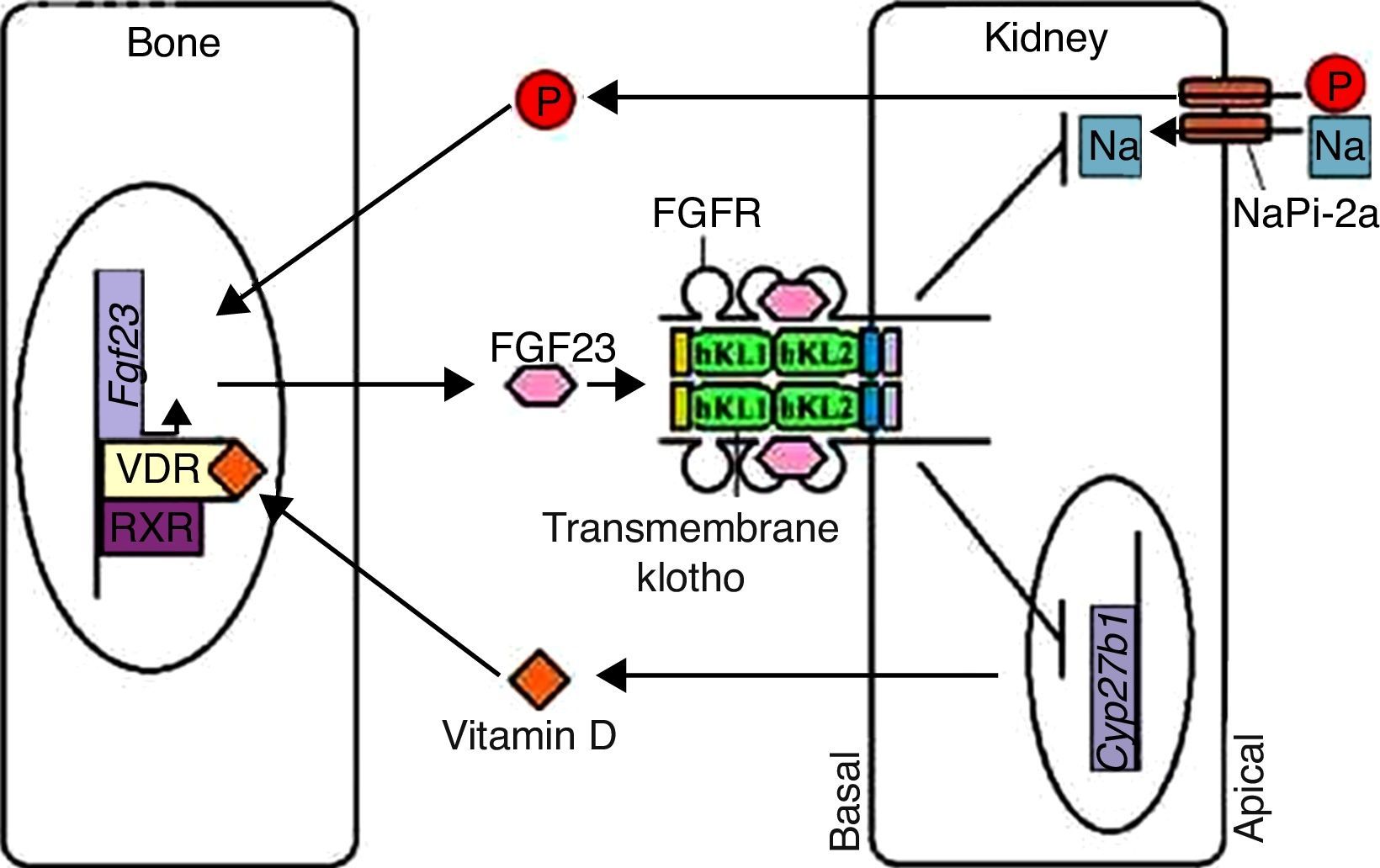
Klotho participates in the regulation of the bone-kidney endocrine axisThe phenotypic similarity between kl−/− mice and mice with reduced Fgf23 expression (Fgf23−/− mice) suggests a functional relationship between Klotho and FGF23. The Klotho transmembrane protein is a required co-factor/co-receptor for FGF23 signaling (Figure 1), and the inactivation of the Klotho-FGF23 axis in kl−/−/Fgf23−/− mice results in increased expression of co-transporter sodium-phosphate type-2a (NaPi-2a).14 FGF23, in turn, is a bone-derived hormone that acts by inhibiting kidney phosphate reabsorption and vitamin D biosynthesis. FGF23, incorporated into the ternary complex of FGF23-FGFR-Klotho, inhibits NaPi-2a activity, increasing phosphaturic activity. Therefore, FGF23 and Klotho act on the bone-kidney endocrine axis to maintain phosphate homeostasis (Figure 2).4,14,20,21
Phosphate and vitamin D homeostasis regulation. Vitamin D upon binding to its vitamin D receptor (VDR) in osteocytes induces VDR-retinoid X receptor (RXR) heterodimer binding, which activates Fgf23 expression. Phosphate also induces a similar reaction. FGF23 then binds to the FGFR-Klotho complex in renal tubular cells, blocking phosphate reabsorption by NaPi-2a transporters and vitamin D synthesis by suppressing the Cyp27b1 expression and upregulating Cyp24 expression. This mechanism controls the phosphate and vitamin D levels in the body; the Fgf23 expression increases when the levels of these two factors are elevated. After re-establishing normal levels, Fgf23 expression is downregulated and maintains phosphate reabsorption and vitamin D synthesis.
This bone-kidney endocrine axis also regulates vitamin D levels. High serum phosphate levels increase Fgf23 expression in the bone. FGF23 represses Cyp27b1 expression and increases Cyp24 expression, reducing 1α-hydroxylase levels. The end result is the reduction of 1.25(OH)2D3 serum synthesis (Figure 2). These mechanisms are essential for vitamin D homeostasis, preventing hypervitaminosis D. It has also been found that the administration of 1.25(OH)2D3 induces the expression of Klotho in the kidney, which reinforces the integrated mechanism between FGF23 and Klotho in this axis.4,22
Disturbances of these negative feedback loops can also lead to a hypercalcemic state because vitamin D also promotes gut calcium absorption.4,23,24
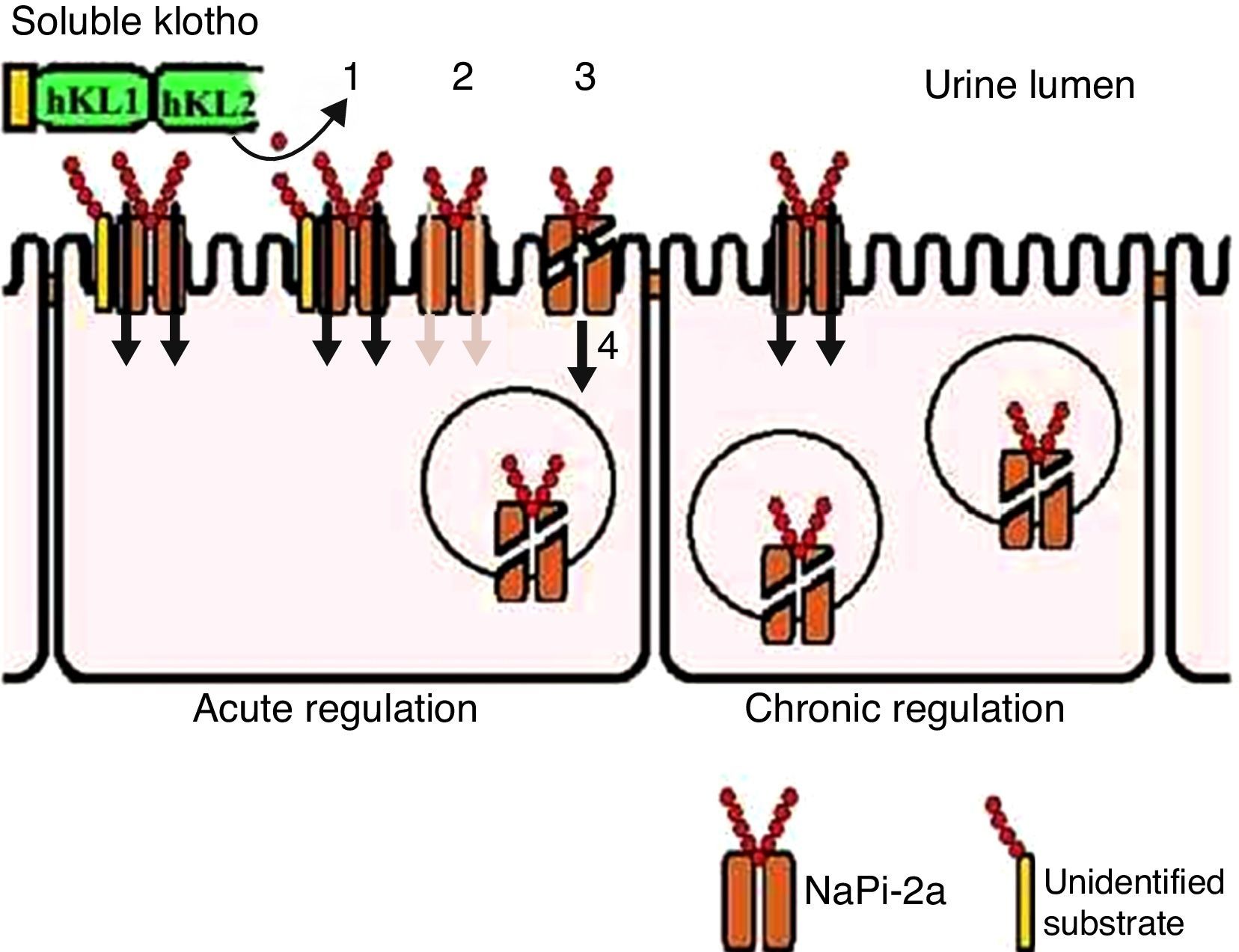
From observations of Fgf23−/− mice, there is additional phosphaturic activity from secreted Klotho protein that is FGF23 independent. The Klotho glycosidase activity acts on an unknown substrate that is present in the brush border of kidney proximal tubular cells. This modification is accompanied by proteolytic cleavage (this step occurs, but it is not required to inactivate the transporter) and NaPi-2a co-transporter internalization (Figure 3).2
Soluble Klotho protein acts on phosphate transport in the proximal tubules. In acute regulatory conditions, Klotho modifies an unknown substrate, glycan (1), reducing the phosphate transporter coupled to sodium (2). Additionally, the carrier undergoes proteolytic cleavage (3) and subsequent endocytosis (4). Upon sustained elevated soluble Klotho levels or chronic regulation conditions, there is ongoing reduction of these transporters at the cell surface.
Klotho can also inhibit phosphate transport in vascular smooth muscle cells through NaPi class 3 transporters, Pit-1 and Pit-2, preventing vascular calcification events, because excessive phosphate influx in these cells promotes a cascade of events responsible for the calcium and phosphate mineralization in their interior.25
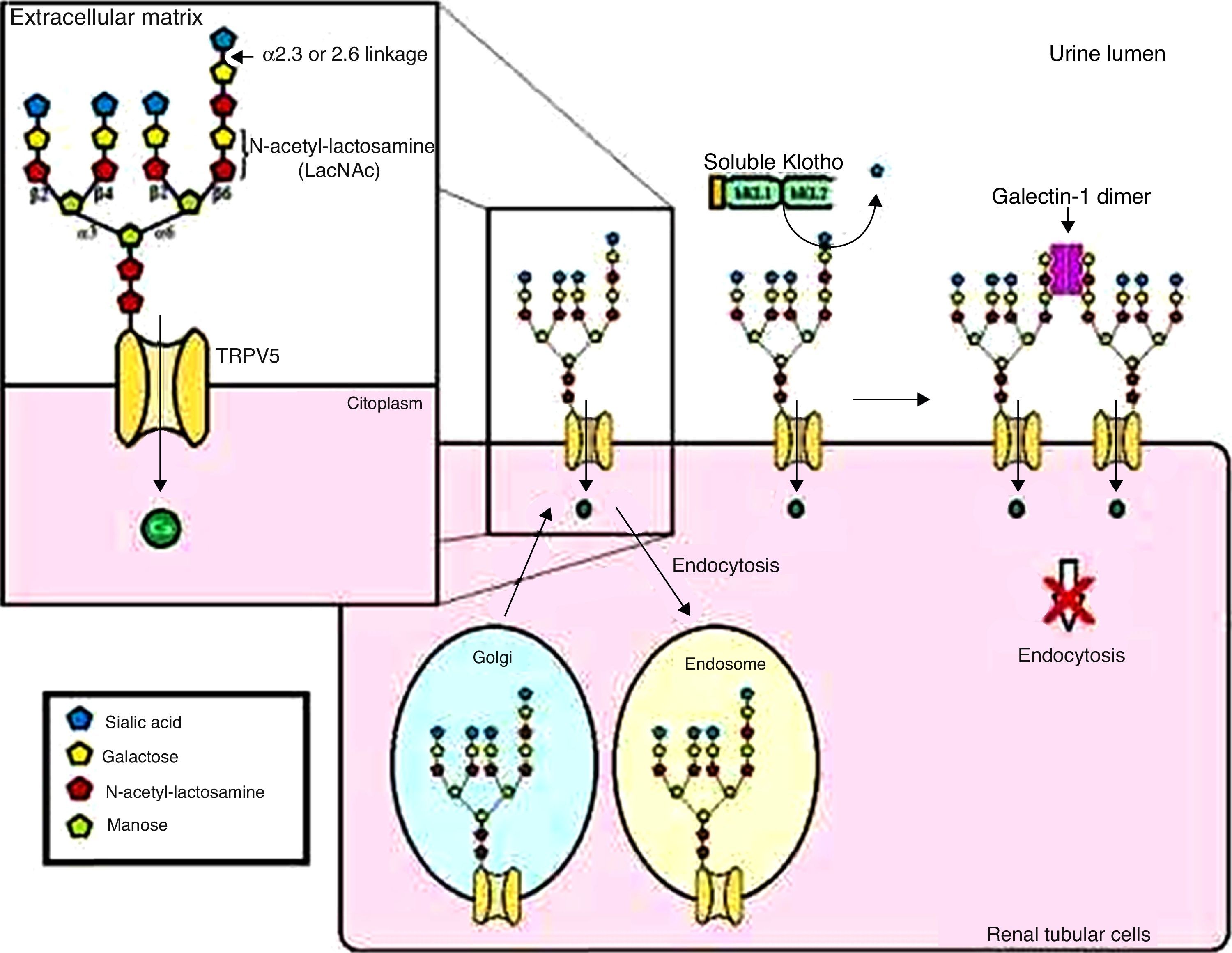
Effect of the Klotho protein on ion channel regulationThe secreted Klotho protein regulates other ion channels, such as the transient receptor potential cation channel, member 5 of subfamily V (TRPV5), which is primarily responsible for Ca2+ entry during kidney transepithelial reabsorption. Secreted Klotho protein inhibits TRPV5 internalization through its sialidase activity on this ion channel, increasing TRPV5 cell surface levels and with this the Ca2+ inflow and kidney reabsorption (Figure 4).4,26,27
General model for TRPV5 regulation by Klotho. Soluble Klotho removes the sialic acid residue attached by α2.6 bonding in the N-acetyl-lactosamine (LacNAc) repeats of the TRPV5 N-glycans. The LacNAc, when exposed, can bind to its ligand, the galectin-1 dimer, in the extracellular matrix. This mechanism reduces carrier internalization.
The retaining mechanism of the cell surface ion channel occurs during the regulation of potassium channels in the kidney outer medulla (ROMK1), resulting in an increase in ROMK1 in the plasma membrane of renal tubular cells, with increased potassium secretion in urine.28
Klotho as an insulin/growth factor insulin-like 1 signaling mechanism regulatorKlotho protein is associated with insulin/growth factor insulin-like 1 (IGF-1) signaling pathway regulation, which results in oxidative stress suppression. Transcription factors known as nuclear mammalian forkhead box O (FOXO) are factors that directly bind to antioxidant enzyme promoters, such as catalase and mitochondrial manganese-superoxide dismutase (SOD2), inducing enzyme expression. As a result, there is increased removal of reactive oxygen species (ROS), conferring oxidative stress resistance. These FOXOs are negatively regulated by enabling insulin/IGF-1 signaling, which promotes the serine-threonine kinase Akt phosphorylation. The Akt has the ability to phosphorylate FOXO, resulting in its exclusion from the nucleus and inactivation. Klotho protein acts by inhibiting insulin/IGF-1 signaling, decreasing FOXO phosphorylation and increasing SOD2 expression and minimizing oxidative stress.11,29
Effect of Klotho protein on the vascular endotheliumBoth secreted Klotho proteins have anti-apoptotic and anti-aging activity on vascular endothelial cells. These cells are continuously exposed to Klotho. The vascular endothelium, due to the release of nitric oxide (NO) in response to specific agonists such as acetylcholine, plays an important role in vascular tone maintenance. It has been shown that mutations in the Klotho gene significantly attenuate endothelium-dependent vasodilation of the aorta and arterioles in response to acetylcholine.30,31 There is evidence that Klotho in the secreted and membrane bound forms can up-regulate NO production, although the mechanisms are still unknown.4,11 It is believed that defects in the Klotho gene down-regulate endothelium NO synthase (eNOS). The effects of Klotho on the vascular endothelium are protective against endothelial dysfunction.
Kl−/− mice subjected to the induction of lower limb ischemia exhibited persistent blood flow loss and decreased capillary capacity, in contrast to the high perfusion observed in heterozygous Klotho animals (kl+/−) and wild-type animals. Kl−/− animals present with deficient angiogenesis and reduced levels of urinary NO and tissue cyclic guanosine monophosphate (cGMP), with high rates of progression to spontaneous amputation.32 Moreover, the Klotho protein is capable of increasing angiotensin-converting enzyme-I activity in endothelial cells by the activation of the cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) pathway,33 suggesting involvement of the renin-angiotensin system and NO in vascular tone maintenance.
The Klotho protein has an anti-apoptotic effect on human umbilical vein endothelial cells, with decreased caspase-3 and caspase-9 activity, therefore acting as a humoral factor. It retards cellular aging by mechanisms involving p53/p21.34 Furthermore, from its ability to bind several members of the Wnt family, Klotho can suppress biological activity, working as an antagonist to prevent accelerated cellular aging.13
The Klotho protein has been assessed as a therapeutic tool in particular in respect to preventing activity of age-related phenotypes.30,35,36 The main aim of this review is to describe the possible role of the Klotho protein as a biomarker in sickle cell disease.
Klotho protein in sickle cell diseaseSome studies have evaluated the association of Klotho SNPs with subphenotypes of individuals with SCA, one type of SCD.15–19 It is a disorder of monogenic autosomal recessive inheritance, characterized by a mutation in the HBB gene, where valine replaces glutamic acid in the β-globin polypeptide chain. This mutation results in the variant hemoglobin named S (Hb S), which, under deoxygenated conditions, tends to polymerize inside the red blood cell conferring a sickle shape.
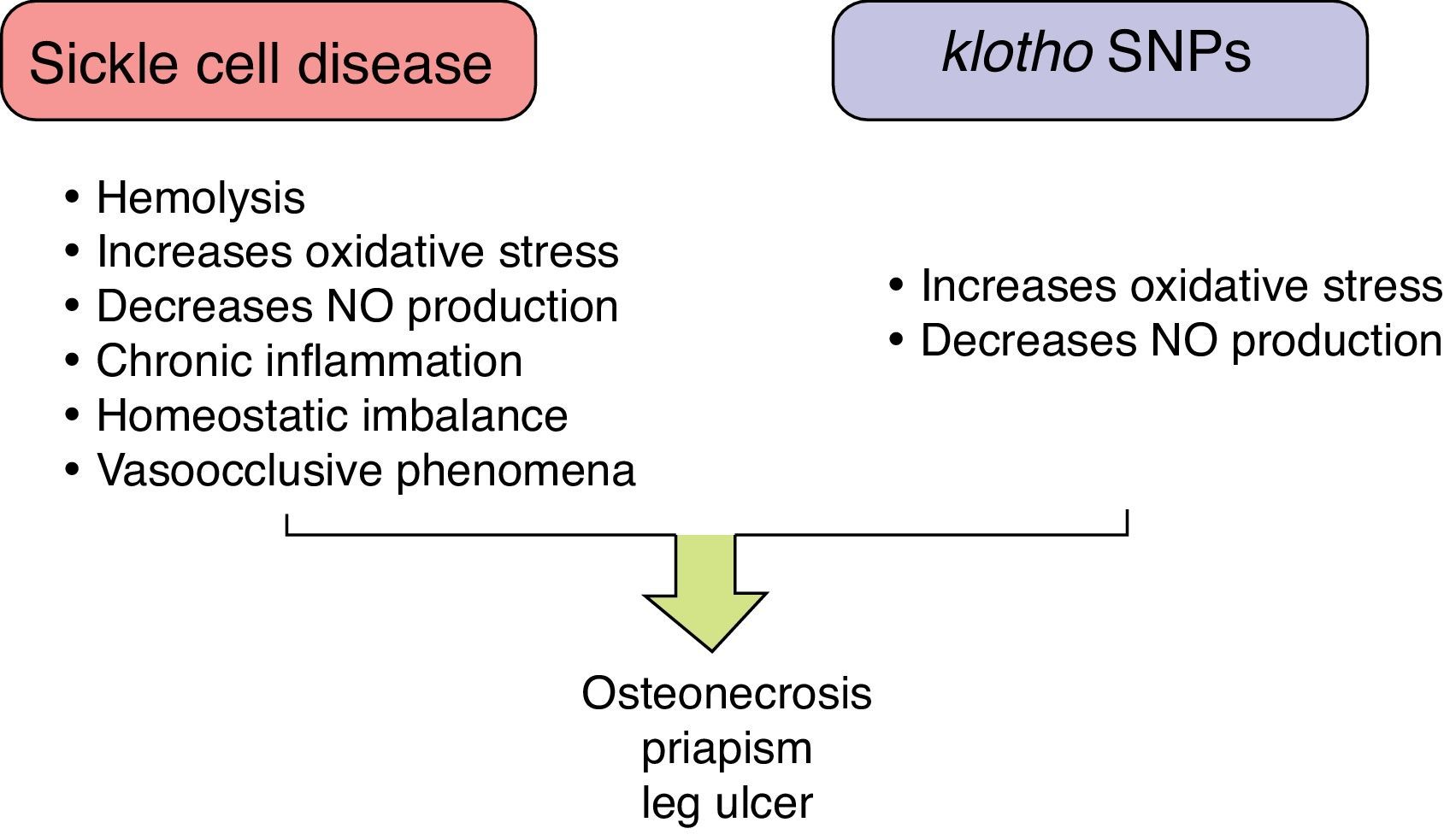
These individuals exhibit a chronic inflammatory and hemolytic state, with significant production of ROS, increased molecule adhesion of endothelial cells and blood cells, and reduced NO production resulting in vaso-occlusive phenomena; all of these are important events that trigger the varied subphenotypes of the disease.37 The large phenotypic variability observed in SCD (including pain crises, stroke, priapism, osteonecrosis, leg ulcers, bacteremia, pulmonary hypertension, acute chest syndrome, and gallstones) demonstrates gene interactions in subphenotype development.38
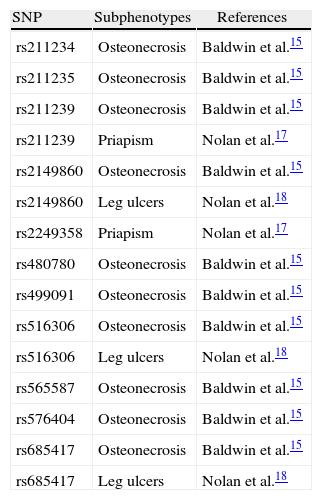
Individuals with SCA and osteonecrosis of the hip or shoulder, with or without α-thalassemia, showed that 10 Klotho SNPs were significantly associated with these subphenotypes.15 Additionally, two SNPs have been associated with priapism17 and three with leg ulcers18 (Table 1). These associations may be underestimated, given that control group individuals could develop such subphenotypes in the future.
A list of Klotho SNPs significantly associated with sickle cell disease subphenotypes.
| SNP | Subphenotypes | References |
| rs211234 | Osteonecrosis | Baldwin et al.15 |
| rs211235 | Osteonecrosis | Baldwin et al.15 |
| rs211239 | Osteonecrosis | Baldwin et al.15 |
| rs211239 | Priapism | Nolan et al.17 |
| rs2149860 | Osteonecrosis | Baldwin et al.15 |
| rs2149860 | Leg ulcers | Nolan et al.18 |
| rs2249358 | Priapism | Nolan et al.17 |
| rs480780 | Osteonecrosis | Baldwin et al.15 |
| rs499091 | Osteonecrosis | Baldwin et al.15 |
| rs516306 | Osteonecrosis | Baldwin et al.15 |
| rs516306 | Leg ulcers | Nolan et al.18 |
| rs565587 | Osteonecrosis | Baldwin et al.15 |
| rs576404 | Osteonecrosis | Baldwin et al.15 |
| rs685417 | Osteonecrosis | Baldwin et al.15 |
| rs685417 | Leg ulcers | Nolan et al.18 |
However, other studies have not succeeded in replicating these results. Ulug et al.19 tested Klotho SNPs in 39 SCD patients who had femoral and humeral head avascular necrosis, evidenced by symptoms and imaging studies. The controls were 205 individuals with SCD without symptoms of this subphenotype. There was no reproduction of the association found by Baldwin et al.15 which may be explained by the inadequate sample size and by the absence of any radiological investigation of controls as it is not possible to ensure that the control individuals were not asymptomatic patients in early stages of avascular necrosis. Elliotte et al.16 tested the association of Klotho SNPs and priapism in SCD patients, but were unsuccessful in finding the association between SNPs and subphenotypes reported by Nolan et al.17 probably due to differences in the definition of priapism, the patients’ ages and adjustments made in the tests.
Osteonecrosis, associated with vaso-occlusive events and increased blood viscosity, is a common clinical manifestation of SCD. Bone microcirculation is a favorable environment for sickled red blood cells, leukocytes, and platelet deposition, leading to infarction and necrosis of bone tissue.38,39 So, despite the methodological differences that did not allow reproducibility of the results of Baldwin et al.15 it may be that endothelial dysfunction is exacerbated in individuals with SCD and Klotho SNPs due to the loss of the protective effect against oxidative stress as well as reductions in the production of NO that affect vascular tone.
Priapism is a prolonged penile erection irrespective of sexual interest, and has a direct relation with intravascular hemolysis.38 Normal erection is dependent on the activation of guanylate cyclase by NO for the synthesis of cGMP, which generates the rapid relaxation of penile smooth muscle. Erection regulation is driven by phosphodiesterase type 5, which controls the production of cGMP.40,41 However, the expression of phosphodiesterase type 5 is reduced when there is NO depletion. Both hemolysis and genetic alterations in Klotho expression contribute to the reduction in NO synthesis, which affects the erection control mechanisms by phosphodiesterase type 5, thereby prolonging erection.
Similar to priapism, leg ulcers are related to the severity of hemolysis, and both NO depletion and oxidative stress appear to play a role in the development of ulcers.18,38 However, the pathophysiology of this clinical event still needs to be elucidated in order to clarify whether these mechanisms cause leg ulcers when Klotho SNPs are present.
Figure 5 summarizes the role of Klotho SNPs to establish the SCD subphenotypes.
ConclusionThe Klotho gene has a wide range of functions in several structures of the body, increasing phosphaturic activity and reducing 1.25(OH)2D3 synthesis, regulating ion channel levels on the cell surface with anti-aging and anti-apoptotic effects, reducing oxidative stress and inducing the production of NO. SNPs in this gene have reportedly been associated with subphenotypes of SCD, however this data was not reproduced probably due to methodological differences. Given the endothelial involvement of the Klotho protein and knowledge about SCD pathophysiology (markedly centered around hemolysis and vaso-occlusive phenomena), it is essential to conduct further studies with the power needed to test the associations between the Klotho gene and SCD, in order to identify genetic markers of risk in these individuals and allow earlier and more effective therapeutic interventions.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by grants from the Brazilian National Council of Research (CNPq) (311888/2013-5) (M.S.G.); the Foundation of Research and Extension of Bahia (FAPESB) (3626/2013) (M.S.G.); PPSUS/FAPESB (020/2013 EFP_00007295), (M.S.G.), and CNPq (402022/2010-6) (coordinated by F.F.C.). The sponsors of this study are public or nonprofit organizations that support science in general, and they had no role in gathering, analyzing, or interpreting the data.