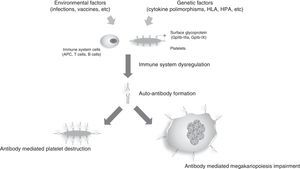
Primary immune thrombocytopenic purpura (ITP) is an acquired immune-mediated disorder characterized by transient or persistent decreased platelet count (<100×109/L) that affects children and adults in the absence of other underlying causes.1,2 The low platelet count results from platelet destruction by antiplatelet autoantibodies associated to causes such as insufficient platelet production, which is also related to these autoantibodies, and T cell immune dysregulation (Figure 1).2
Simplified representation of ITP mechanisms. Immune dysregulation caused by environmental factors, such as viral infections and vaccines, associated to genetic factors (cytokine polymorphisms, HLA, HPA, etc.) results in autoantibody production that mainly recognize surface glycoproteins such as GpIIb-IIIa, GpIb-IX and GpIa-IIa which are present on the surface of platelets and megakaryocytes. These autoantibodies lead to platelet destruction and decreased platelet production, resulting in thrombocytopenia and bleeding symptoms.
The presence of the autoantibodies may be demonstrated in the serum of approximately 70% of ITP patients, usually directed against platelet surface glycoproteins (Gp), such as Gp IIb-IIIa, Gp Ib-IX and Gp Ia-IIa.2,3 ITP has an estimated incidence of 5.8 cases in 100,000 young individuals with similar distribution between genders, and 1.6 cases in 100,000 middle aged individuals with higher rates among women (1.9 females: 1 male). This condition may be classified according to duration as newly diagnosed (less than 3 months), persistent (3–12 months) and chronic (more than 12 months).1,2 The clinical features are usually different in children and adults. In childhood, ITP usually has an abrupt onset, often starting 1–2 weeks after a viral infection or up to six weeks after vaccinations (generally the measles, mumps and rubella – MMR vaccination) with recovery being spontaneous in around 70–80% of the cases within a few weeks regardless of therapy. In adults, the disease has an insidious onset, with no preceding illness and frequently it has a chronic course.4 Clinical manifestations of ITP are usually a consequence of the severe thrombocytopenia leading to bleeding symptoms. Hemorrhagic events are highly heterogeneous but patients frequently present mild mucocutaneous bleeding.5,6 Major bleeding risk is low in pediatric patients but in adults, additional modifiers such as existing comorbidities, age, activities and medications may affect the risk of significant bleeding.6 The main goal of ITP treatment is to raise the platelet count to control bleeding symptoms and to prevent major hemorrhage as well as to minimize treatment toxicity and maximize quality of life.6 The treatment is rarely indicated when the platelet count is above 20×109/L in the absence of other factors such as major bleeding.6 First line therapy is generally based on the use of corticosteroids with an initial response rate of approximately 80% or intravenous immunoglobulin (IgIV or anti-D in Rh positive patients), when a rapid increase in the platelet count is needed, with a transient response in approximately 80% of cases.6 When the patient fails to respond or relapses after first-line therapy, the second-line treatment used generally consists of immunosuppressive agents, rituximab, splenectomy and more recently thrombopoietin receptor antagonists (TRA).2,6 TRA (romiplostim, eltrombopag) are new agents that have very high efficacy and few side effects but are expensive and still need to be evaluated over a longer follow-up.6,7
Although the comprehension of this complex autoimmune disorder has developed greatly since its first description in the middle of last century,8 mechanisms and factors leading to ITP are not yet fully understood.2,6 Environmental and genetic factors, such as cytokine gene polymorphisms, human leukocyte antigens (HLAs) and human platelet antigens (HPAs) seem to play a role.9–12 HPAs are polymorphisms of platelet surface Gps which result in alloantigen expression, leading to alloimmunization mainly after transfusions and pregnancies.13 These polymorphisms have heterogeneous distribution in populations with distinct ethnic backgrounds and especially HPA-2 and HPA-5 have been described to be possible risk factors in the development of ITP.10,11,14,15 In this issue, Carmo et al.16 have evaluated the association of HPAs and chronic ITP (cITP) in a small group of patients from the Amazonian region of Brazil, which has a population with a particular HPA distribution compared to the Brazilian population in general.17 It is important to note that ITP is not a common disease and it is difficult for a single center study to enroll a large number of patients. The authors found a higher incidence of the HPA-1a, HPA-3b and HPA-5b alleles in cITP compared to healthy blood donors from the same region, but they found no involvement of HPA-2 in this group of patients. These results differ from data obtained in other studies in which the HPA-1 and HPA-3 systems seem to have no association with ITP in the populations studied, but HPA-2 and HPA-5 were associated.10–12,15 The mechanisms that explain these associations are not known yet. Although Carmo et al.16 evaluated a small group of patients from a single center, the results are interesting and further enhance the concept that ITP is a complex autoimmune disorder with mechanisms that need extensive effort to understand better and provide new and more efficient treatment possibilities.
Conflicts of interestThe authors declare no conflicts of interest.
See paper by do Carmo et al. in Rev Bras Hematol Hemoter. 2017;39(2):122–6.