Intravascular hemolysis can occur in a number of diseases, including sepsis, malaria and the hemolytic anemias [sickle cell disease (SCD), paroxysmal nocturnal hemoglobinuria, etc.], as well as following certain events such as mismatched transfusions. The pathophysiological significance of intravascular hemolysis had been largely ignored in diseases such as SCD save for its effect on red cell numbers, however, relatively recent studies have shed light on the major effects that hemolytic processes have on vascular biology, particularly on the endothelium, inflammatory processes and oxidative stress.
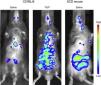
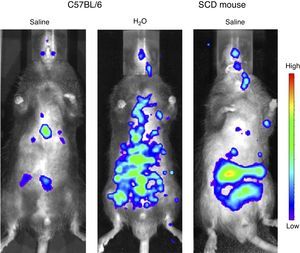
Under normal circumstances, hemoglobin (Hb) is contained in the red blood cells (RBCs), and employed primarily for oxygen transport. However, the destruction of RBC (i.e. hemolysis) results in the release of Hb into the circulation. If this hemoglobin is not neutralized immediately into less toxic metabolites by specialized scavenger proteins, such as haptoglobin and hemopexin, as can occur when extensive hemolysis overwhelms innate protective mechanisms, this cell-free Hb causes chaos.1 The potential extent of this damage was perhaps first recognized when Reiter et al.2 showed that the decompartmentalization of Hb into the plasma significantly impairs vascular nitric oxide (NO) bioavailability, potentially facilitating vasoconstriction and alterations in blood flow, as well as inducing endothelial dysfunction and platelet activation.3,4 While the contribution of hemolysis and subsequent NO depletion to manifestations of SCD, such as pulmonary hypertension, has been disputed,5 more recent evidence has highlighted the significant effects that intravascular hemolysis has on inflammatory processes. The induction of acute hemolytic events in C57BL/6 mice (using intravenous water injections, resulting in plasma levels of cell-free Hb that were similar to those seen in mice with SCD) was found to induce a rapid and extensive systemic and vascular inflammatory response (within 15min). This is possibly mediated by vascular NO consumption, and leads to extensive systemic inflammation (Figure 1) and leukocyte recruitment to the blood vessels.6 Given the evidence that inflammation and leukocyte adhesion to the endothelium can initiate and propagate vaso-occlusive processes,7,8 it is reasonable to conclude that vascular inflammatory processes that are triggered by acute intravascular hemolytic events may be of pathophysiological significance in SCD as well as in the other diseases or medical events in which they occur.
Quantification of systemic inflammation in vivo using the IVIS Lumina System® (Caliper LifeSciences, MA). Images obtained 15min after intravenous injections of saline or water (150μL) in C57BL/6 mice and a chimeric sickle cell disease mouse. A chemiluminescent probe (XenoLight Rediject Inflammation Probe; Perkin Elmer, MA) was injected in the mouse to quantify myeloperoxidase production of activated phagocytes and neutrophils. Similar data were originally reported in reference 6.
Once oxyhemoglobin has reacted with NO in the blood vessel, Hb-Fe3+ is formed and can accumulate both in the circulation and in tissues.9 In a secondary Hb-mediated toxic mechanism, Hb-Fe3+ releases damaging hemin, a hydrophobic molecule shown to induce neutrophil extracellular trap (NET) production, stimulate inflammasome formation and cause toll-like receptor (TLR)-4-mediated vaso-occlusion in mice with SCD, as well as lipoprotein oxidation.10–13 These secondary inflammatory and oxidative effects of hemin appear to occur in a less immediate,6 but probably more sustained manner, causing tissue damage and endothelial dysfunction, as well as sustaining leukocyte activation in the blood vessels.9
Hemolysis, therefore, represents a major disease mechanism and failure to neutralize Hb after its release from the RBC can result in vascular inflammation and organ dysfunction, potentially contributing to clinical complications that have been associated with hemolytic diseases, such as priapism, pulmonary hypertension and leg ulcers.14
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to Lucas Eduardo Botelho de Souza and Prof. Dr. Dimas Tadeu Covas for assistance with IVIS experiments depicted herein. The IVIS experiments were funded by FAPESP (grants 2008/50582-3; 11/50959-7 [CBA]).