Hemolytic disease of the fetus and newborn is a public health problem caused by maternal-fetal incompatibility; no prophylaxis is available for most alloantibodies that induce this disease. This study reviews the literature regarding which antibodies are the most common in maternal plasma and which were involved in hemolytic disease of the fetus and newborn.
MethodSeventy-five studies were included in this review using a systematic search. Two independent authors identified studies of interest from the PubMed and SciELO databases.
Main resultsForty-four case reports were identified, of which 11 babies evolved to death. From 17 prevalence studies, the alloimmunization rate was 0.17 % with 161 babies receiving intrauterine transfusions and 23 receiving transfusions after birth. From 28 studies with alloimmunized pregnant women (7616 women), 455 babies received intrauterine transfusions and 21 received transfusions after birth.
ConclusionRh, Kell, and MNS were the commonest blood systems involved. The geographical distribution of studies shows that as these figures vary between continents, more studies should be performed in different countries. Investing in early diagnosis is important to manage the risks and complications of hemolytic disease of the fetus and newborn.
Hemolytic disease of the fetus and newborn (HDFN) is caused by maternal-fetal incompatibility when the mother has an antibody (IgG subclass) against an antigen expressed on the fetal red blood cell (RBC) and this antibody crosses the placenta.1 Alloantibodies usually form after exposure to non-self-antigens due to a transfusion or transplantation, or when a pregnancy results in sensitization creating antibodies against RBC antigens.2 HDFN has different degrees of complications that can be classified as mild, moderate, or severe. The physiopathology results in various complications such as anemia, aplastic anemia, hyperbilirubinemia, fetal hydrops, kernicterus, and death.3
Different maternal alloantibodies can cause HDFN, the most common are anti-D and other Rh antibodies, and Kell.1 However, antibodies against high prevalence antigens can also cause severe HDFN, as in the case report described by Levitt et al.4 The administration of prophylactic anti-D immunoglobulin in RhD negative women after delivery of a RhD positive child significantly reduced the incidence of HDFN related to anti-D in high-income countries after the 1960s.1,5 However, in less developed countries, HDFN still is a significant problem.6 Pregnant women classified as RhD positive for the D variant (partial D phenotype) who do not receive immunoglobulin prophylaxis may develop anti-D antibodies.7
The International Society of Blood Transfusion (ISBT) recognizes 43 different blood group systems and 378 RBC antigens of which 345 are in the blood group system.8 No prophylaxis is available for antibodies from other blood groups.1 Thus, identifying the antibodies that cause HDFN and the most common alloantibodies in populations could direct new research and the development of alloantibody prophylaxis. In addition, it is important to monitor fetuses and babies with any chance of developing HDFN and correctly identify the alloantibodies involved in HDFN. This study reviews the literature regarding which antibodies are the most common in maternal plasma and which are involved in hemolytic disease of the fetus and newborn.
Material and methodsEligibility criteria and literature searchA systematic search performed by two independent authors extracted studies from the MEDLINE (accessed by PubMed) and Scientific Electronic Library Online (SciELO) databases. The terms used in the search were ‘hemolytic disease of the fetus and newborn’, ‘alloimmunization’, ‘isoimmunization’, ‘hydrops fetalis’, ‘fetal hemolytic anemia’ using the functions ‘AND’ and ‘OR’. Additionally, the exact words and synonyms were used as text words for searches in titles and abstracts. The search was restricted to studies published between 2013 and 2023. A total of 1690 results were found.
After reading the title and abstract, 1589 studies were excluded. After reading these papers, 75 were selected for data collection. To be considered eligible, case reports should present at least one case of HDFN (presence of alloantibodies causing anemia) identified by the study authors. For other study types (cross-sectional, case-control, cohort), the presence of maternal alloimmunization was accepted even if the study did not mention the clinical outcome of the babies or the development of HDFN but only mentioned the risk of HDFN. Case reports without HDFN were excluded even if alloimmunization was present. Case-control, cross-sectional and cohort studies were excluded when alloantibodies were not identified and when the study was only about phenotyping and/or genotyping pregnant women without alloimmunization.
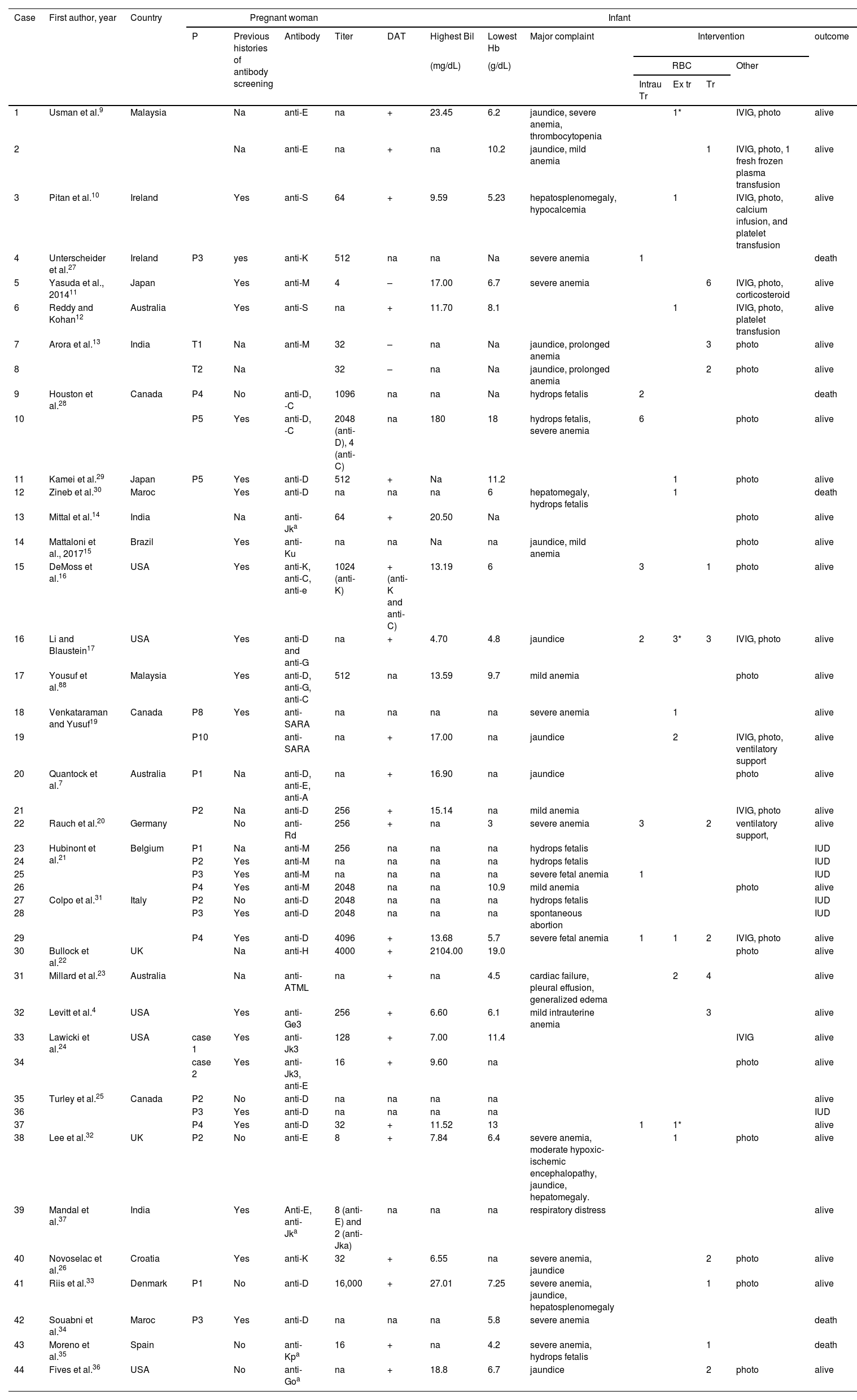
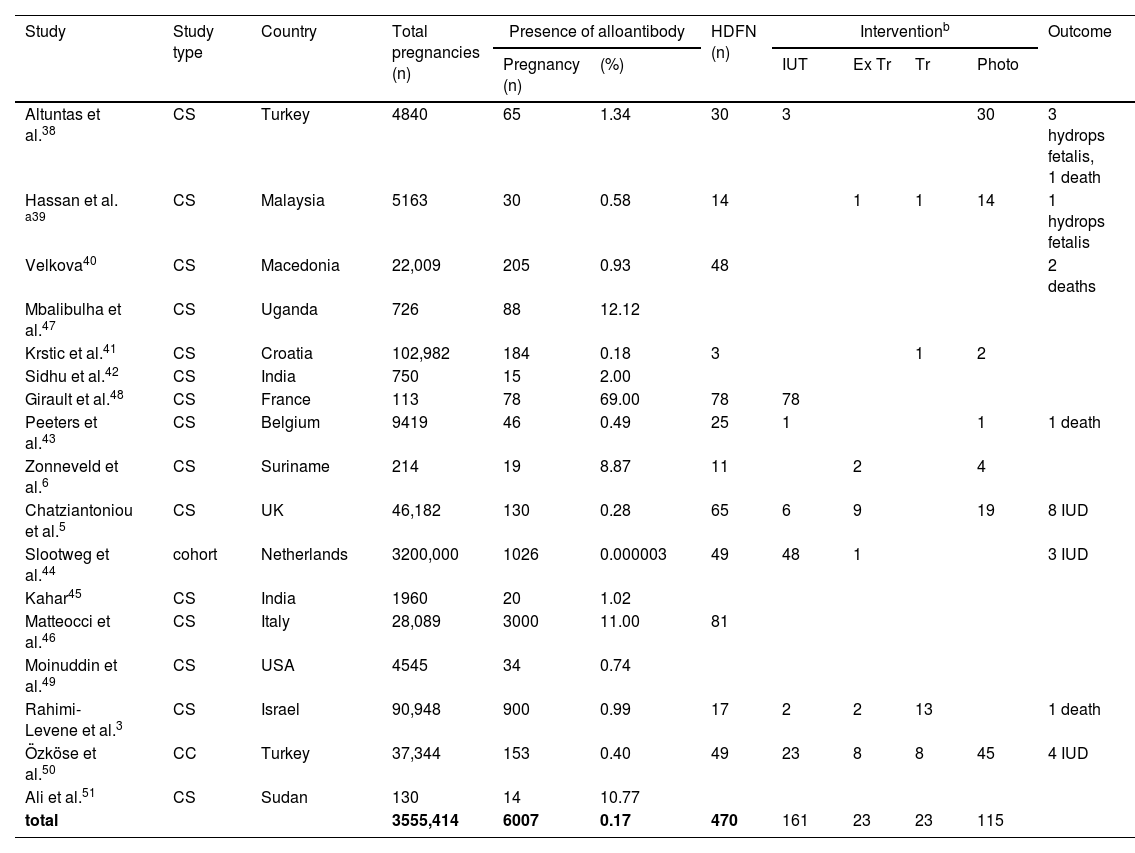
ResultsTable 1 shows the data collected from case-report studies,4,7,9–37Table 2 presents data from prevalence studies,3,5,6,38–51 and Table 3 presents data from alloimmunized pregnant women.1,11,52–77 A total of 75 studies were reviewed, 31 case reports, 19 cohorts, 22 cross-sectional, and three case-control studies. One study was classified both as a case report and a review and was included in both Tables 1 and 3.
RBC alloantibodies in case-report studies.
| Case | First author, year | Country | Pregnant woman | Infant | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | Previous histories of antibody screening | Antibody | Titer | DAT | Highest Bil | Lowest Hb | Major complaint | Intervention | outcome | ||||||
| (mg/dL) | (g/dL) | RBC | Other | ||||||||||||
| Intrau Tr | Ex tr | Tr | |||||||||||||
| 1 | Usman et al.9 | Malaysia | Na | anti-E | na | + | 23.45 | 6.2 | jaundice, severe anemia, thrombocytopenia | 1* | IVIG, photo | alive | |||
| 2 | Na | anti-E | na | + | na | 10.2 | jaundice, mild anemia | 1 | IVIG, photo, 1 fresh frozen plasma transfusion | alive | |||||
| 3 | Pitan et al.10 | Ireland | Yes | anti-S | 64 | + | 9.59 | 5.23 | hepatosplenomegaly, hypocalcemia | 1 | IVIG, photo, calcium infusion, and platelet transfusion | alive | |||
| 4 | Unterscheider et al.27 | Ireland | P3 | yes | anti-K | 512 | na | na | Na | severe anemia | 1 | death | |||
| 5 | Yasuda et al., 201411 | Japan | Yes | anti-M | 4 | – | 17.00 | 6.7 | severe anemia | 6 | IVIG, photo, corticosteroid | alive | |||
| 6 | Reddy and Kohan12 | Australia | Yes | anti-S | na | + | 11.70 | 8.1 | 1 | IVIG, photo, platelet transfusion | alive | ||||
| 7 | Arora et al.13 | India | T1 | Na | anti-M | 32 | – | na | Na | jaundice, prolonged anemia | 3 | photo | alive | ||
| 8 | T2 | Na | 32 | – | na | Na | jaundice, prolonged anemia | 2 | photo | alive | |||||
| 9 | Houston et al.28 | Canada | P4 | No | anti-D, -C | 1096 | na | na | Na | hydrops fetalis | 2 | death | |||
| 10 | P5 | Yes | anti-D, -C | 2048 (anti-D), 4 (anti-C) | na | 180 | 18 | hydrops fetalis, severe anemia | 6 | photo | alive | ||||
| 11 | Kamei et al.29 | Japan | P5 | Yes | anti-D | 512 | + | Na | 11.2 | 1 | photo | alive | |||
| 12 | Zineb et al.30 | Maroc | Yes | anti-D | na | na | na | 6 | hepatomegaly, hydrops fetalis | 1 | death | ||||
| 13 | Mittal et al.14 | India | Na | anti-Jka | 64 | + | 20.50 | Na | photo | alive | |||||
| 14 | Mattaloni et al., 201715 | Brazil | Yes | anti-Ku | na | na | Na | na | jaundice, mild anemia | photo | alive | ||||
| 15 | DeMoss et al.16 | USA | Yes | anti-K, anti-C, anti-e | 1024 (anti-K) | + (anti-K and anti-C) | 13.19 | 6 | 3 | 1 | photo | alive | |||
| 16 | Li and Blaustein17 | USA | Yes | anti-D and anti-G | na | + | 4.70 | 4.8 | jaundice | 2 | 3* | 3 | IVIG, photo | alive | |
| 17 | Yousuf et al.88 | Malaysia | Yes | anti-D, anti-G, anti-C | 512 | na | 13.59 | 9.7 | mild anemia | photo | alive | ||||
| 18 | Venkataraman and Yusuf19 | Canada | P8 | Yes | anti-SARA | na | na | na | na | severe anemia | 1 | alive | |||
| 19 | P10 | anti-SARA | na | + | 17.00 | na | jaundice | 2 | IVIG, photo, ventilatory support | alive | |||||
| 20 | Quantock et al.7 | Australia | P1 | Na | anti-D, anti-E, anti-A | na | + | 16.90 | na | jaundice | photo | alive | |||
| 21 | P2 | Na | anti-D | 256 | + | 15.14 | na | mild anemia | IVIG, photo | alive | |||||
| 22 | Rauch et al.20 | Germany | No | anti-Rd | 256 | + | na | 3 | severe anemia | 3 | 2 | ventilatory support, | alive | ||
| 23 | Hubinont et al.21 | Belgium | P1 | Na | anti-M | 256 | na | na | na | hydrops fetalis | IUD | ||||
| 24 | P2 | Yes | anti-M | na | na | na | na | hydrops fetalis | IUD | ||||||
| 25 | P3 | Yes | anti-M | na | na | na | na | severe fetal anemia | 1 | IUD | |||||
| 26 | P4 | Yes | anti-M | 2048 | na | na | 10.9 | mild anemia | photo | alive | |||||
| 27 | Colpo et al.31 | Italy | P2 | No | anti-D | 2048 | na | na | na | hydrops fetalis | IUD | ||||
| 28 | P3 | Yes | anti-D | 2048 | na | na | na | spontaneous abortion | IUD | ||||||
| 29 | P4 | Yes | anti-D | 4096 | + | 13.68 | 5.7 | severe fetal anemia | 1 | 1 | 2 | IVIG, photo | alive | ||
| 30 | Bullock et al.22 | UK | Na | anti-H | 4000 | + | 2104.00 | 19.0 | photo | alive | |||||
| 31 | Millard et al.23 | Australia | Na | anti-ATML | na | + | na | 4.5 | cardiac failure, pleural effusion, generalized edema | 2 | 4 | alive | |||
| 32 | Levitt et al.4 | USA | Yes | anti-Ge3 | 256 | + | 6.60 | 6.1 | mild intrauterine anemia | 3 | alive | ||||
| 33 | Lawicki et al.24 | USA | case 1 | Yes | anti-Jk3 | 128 | + | 7.00 | 11.4 | IVIG | alive | ||||
| 34 | case 2 | Yes | anti-Jk3, anti-E | 16 | + | 9.60 | na | photo | alive | ||||||
| 35 | Turley et al.25 | Canada | P2 | No | anti-D | na | na | na | na | alive | |||||
| 36 | P3 | Yes | anti-D | na | na | na | na | IUD | |||||||
| 37 | P4 | Yes | anti-D | 32 | + | 11.52 | 13 | 1 | 1* | alive | |||||
| 38 | Lee et al.32 | UK | P2 | No | anti-E | 8 | + | 7.84 | 6.4 | severe anemia, moderate hypoxic-ischemic encephalopathy, jaundice, hepatomegaly. | 1 | photo | alive | ||
| 39 | Mandal et al.37 | India | Yes | Anti-E, anti-Jka | 8 (anti-E) and 2 (anti-Jka) | na | na | na | respiratory distress | alive | |||||
| 40 | Novoselac et al.26 | Croatia | Yes | anti-K | 32 | + | 6.55 | na | severe anemia, jaundice | 2 | photo | alive | |||
| 41 | Riis et al.33 | Denmark | P1 | No | anti-D | 16,000 | + | 27.01 | 7.25 | severe anemia, jaundice, hepatosplenomegaly | 1 | photo | alive | ||
| 42 | Souabni et al.34 | Maroc | P3 | Yes | anti-D | na | na | na | 5.8 | severe anemia | death | ||||
| 43 | Moreno et al.35 | Spain | No | anti-Kpa | 16 | + | na | 4.2 | severe anemia, hydrops fetalis | 1 | death | ||||
| 44 | Fives et al.36 | USA | No | anti-Goa | na | + | 18.8 | 6.7 | jaundice | 2 | photo | alive | |||
DAT, direct antiglobulin test; Bil, bilirubin; Hb, hemoglobin; photo, phototherapy; Intrau Tr, intrauterine transfusion; Ex tr, exchange transfusion; Tr, transfusion; IVIG, intravenous immunoglobulin; +, positive; -, negative; IUD, intrauterine death; P, pregnancy; T, Twin; np, not performed;.
Frequency of RBC alloantibodies in prevalence studies.
| Study | Study type | Country | Total pregnancies (n) | Presence of alloantibody | HDFN (n) | Interventionb | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy (n) | (%) | IUT | Ex Tr | Tr | Photo | ||||||
| Altuntas et al.38 | CS | Turkey | 4840 | 65 | 1.34 | 30 | 3 | 30 | 3 hydrops fetalis, 1 death | ||
| Hassan et al. a39 | CS | Malaysia | 5163 | 30 | 0.58 | 14 | 1 | 1 | 14 | 1 hydrops fetalis | |
| Velkova40 | CS | Macedonia | 22,009 | 205 | 0.93 | 48 | 2 deaths | ||||
| Mbalibulha et al.47 | CS | Uganda | 726 | 88 | 12.12 | ||||||
| Krstic et al.41 | CS | Croatia | 102,982 | 184 | 0.18 | 3 | 1 | 2 | |||
| Sidhu et al.42 | CS | India | 750 | 15 | 2.00 | ||||||
| Girault et al.48 | CS | France | 113 | 78 | 69.00 | 78 | 78 | ||||
| Peeters et al.43 | CS | Belgium | 9419 | 46 | 0.49 | 25 | 1 | 1 | 1 death | ||
| Zonneveld et al.6 | CS | Suriname | 214 | 19 | 8.87 | 11 | 2 | 4 | |||
| Chatziantoniou et al.5 | CS | UK | 46,182 | 130 | 0.28 | 65 | 6 | 9 | 19 | 8 IUD | |
| Slootweg et al.44 | cohort | Netherlands | 3200,000 | 1026 | 0.000003 | 49 | 48 | 1 | 3 IUD | ||
| Kahar45 | CS | India | 1960 | 20 | 1.02 | ||||||
| Matteocci et al.46 | CS | Italy | 28,089 | 3000 | 11.00 | 81 | |||||
| Moinuddin et al.49 | CS | USA | 4545 | 34 | 0.74 | ||||||
| Rahimi-Levene et al.3 | CS | Israel | 90,948 | 900 | 0.99 | 17 | 2 | 2 | 13 | 1 death | |
| Özköse et al.50 | CC | Turkey | 37,344 | 153 | 0.40 | 49 | 23 | 8 | 8 | 45 | 4 IUD |
| Ali et al.51 | CS | Sudan | 130 | 14 | 10.77 | ||||||
| total | 3555,414 | 6007 | 0.17 | 470 | 161 | 23 | 23 | 115 | |||
%, rate of n pregnant with antibody;.
CS: Cross-sectional; CC: Case-control; IUT: intrauterine transfusion; Ex tr: exchange transfusion; Tr: transfusion; Photo: phototherapy; IUD: intrauterine death.
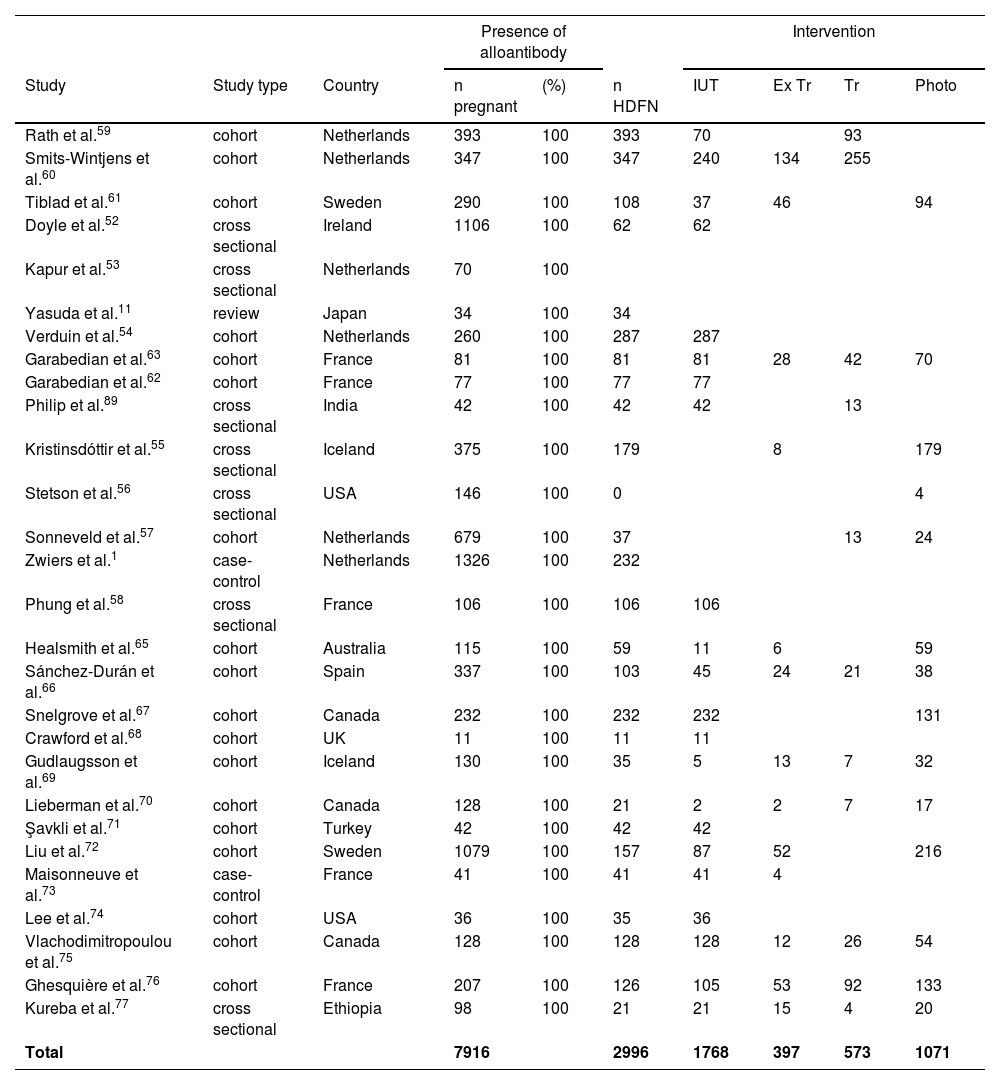
Studies with alloimmunized pregnant women, HDFN, and intervention.
| Presence of alloantibody | Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Study type | Country | n pregnant | (%) | n HDFN | IUT | Ex Tr | Tr | Photo |
| Rath et al.59 | cohort | Netherlands | 393 | 100 | 393 | 70 | 93 | ||
| Smits-Wintjens et al.60 | cohort | Netherlands | 347 | 100 | 347 | 240 | 134 | 255 | |
| Tiblad et al.61 | cohort | Sweden | 290 | 100 | 108 | 37 | 46 | 94 | |
| Doyle et al.52 | cross sectional | Ireland | 1106 | 100 | 62 | 62 | |||
| Kapur et al.53 | cross sectional | Netherlands | 70 | 100 | |||||
| Yasuda et al.11 | review | Japan | 34 | 100 | 34 | ||||
| Verduin et al.54 | cohort | Netherlands | 260 | 100 | 287 | 287 | |||
| Garabedian et al.63 | cohort | France | 81 | 100 | 81 | 81 | 28 | 42 | 70 |
| Garabedian et al.62 | cohort | France | 77 | 100 | 77 | 77 | |||
| Philip et al.89 | cross sectional | India | 42 | 100 | 42 | 42 | 13 | ||
| Kristinsdóttir et al.55 | cross sectional | Iceland | 375 | 100 | 179 | 8 | 179 | ||
| Stetson et al.56 | cross sectional | USA | 146 | 100 | 0 | 4 | |||
| Sonneveld et al.57 | cohort | Netherlands | 679 | 100 | 37 | 13 | 24 | ||
| Zwiers et al.1 | case-control | Netherlands | 1326 | 100 | 232 | ||||
| Phung et al.58 | cross sectional | France | 106 | 100 | 106 | 106 | |||
| Healsmith et al.65 | cohort | Australia | 115 | 100 | 59 | 11 | 6 | 59 | |
| Sánchez-Durán et al.66 | cohort | Spain | 337 | 100 | 103 | 45 | 24 | 21 | 38 |
| Snelgrove et al.67 | cohort | Canada | 232 | 100 | 232 | 232 | 131 | ||
| Crawford et al.68 | cohort | UK | 11 | 100 | 11 | 11 | |||
| Gudlaugsson et al.69 | cohort | Iceland | 130 | 100 | 35 | 5 | 13 | 7 | 32 |
| Lieberman et al.70 | cohort | Canada | 128 | 100 | 21 | 2 | 2 | 7 | 17 |
| Şavkli et al.71 | cohort | Turkey | 42 | 100 | 42 | 42 | |||
| Liu et al.72 | cohort | Sweden | 1079 | 100 | 157 | 87 | 52 | 216 | |
| Maisonneuve et al.73 | case-control | France | 41 | 100 | 41 | 41 | 4 | ||
| Lee et al.74 | cohort | USA | 36 | 100 | 35 | 36 | |||
| Vlachodimitropoulou et al.75 | cohort | Canada | 128 | 100 | 128 | 128 | 12 | 26 | 54 |
| Ghesquière et al.76 | cohort | France | 207 | 100 | 126 | 105 | 53 | 92 | 133 |
| Kureba et al.77 | cross sectional | Ethiopia | 98 | 100 | 21 | 21 | 15 | 4 | 20 |
| Total | 7916 | 2996 | 1768 | 397 | 573 | 1071 | |||
Results extracted from case reports included 47 cases with antibodies, but three cases were excluded because the babies did not show any signs of HDFN, one with anti-H,22 and two with anti-Jk3.24 Of the 44 cases with HDFN, 26 women had already been screened for antibodies prior to the reported pregnancy. The antibodies implicated as causing HDFN were anti-D (10 cases in isolation and 5 with associated antibodies), anti-E (3 cases in isolation and 3 cases with associated antibodies), anti-M (6 cases), anti-Jk3 (1 in isolation and 1 with associated antibodies), anti-Jka (1 in isolation and 1 with associated antibodies), anti-G (2 cases with associated antibodies), anti-K (2 in isolation and 1 with associated antibodies), anti-S (2 cases), anti-SARA (2 cases), anti-C (4 cases with associated antibodies), anti-e and anti-A (1 case with associated antibodies each) and anti-Ku, anti-Goa, anti-Ge3, anti-Kpa, anti-Rd, anti-ATML and anti-H with each one in isolation. The most observed complications reported were severe anemia (13 cases), mild anemia (6 cases), and jaundice (12 cases). Of the 44 cases, 11 evolved to death. Of the anti-D cases, two women were D partial7,25 responsible for 5 cases of anti-D HDFN. A total of 20 IUTs were performed in 9 fetuses, 16 exchange transfusions in 12 babies and 33 transfusions in 14 babies. Phototherapy were necessary as treatment for 25 patients.
Table 2 presents 17 prevalence studies (15 cross-sectional, 1 cohort, and 1 case-control). A total of 3555,414 pregnant women were evaluated; of these 6007 women presented alloantibodies in the gestational period, corresponding to a 0.17 % alloimmunization rate. HDFN was present in 470 babies, of these 161 babies received at least one IUT, 23 received transfusions, 23 performed exchange transfusions, and 115 received phototherapy. Four babies had hydrops fetalis, and 20 evolved to death (15 intrauterine deaths).
For alloimmunized pregnant women, 28 studies were included (1 review, 2 case-control, 7 cross-sectional, and 18 cohort). A total of 7911 women presented alloantibodies in the gestational period. Almost one third (2996) of the babies had HDFN and of these 1768 babies received at least one IUT, 397 received exchange transfusions, 573 received transfusions, and 1071 received phototherapy.
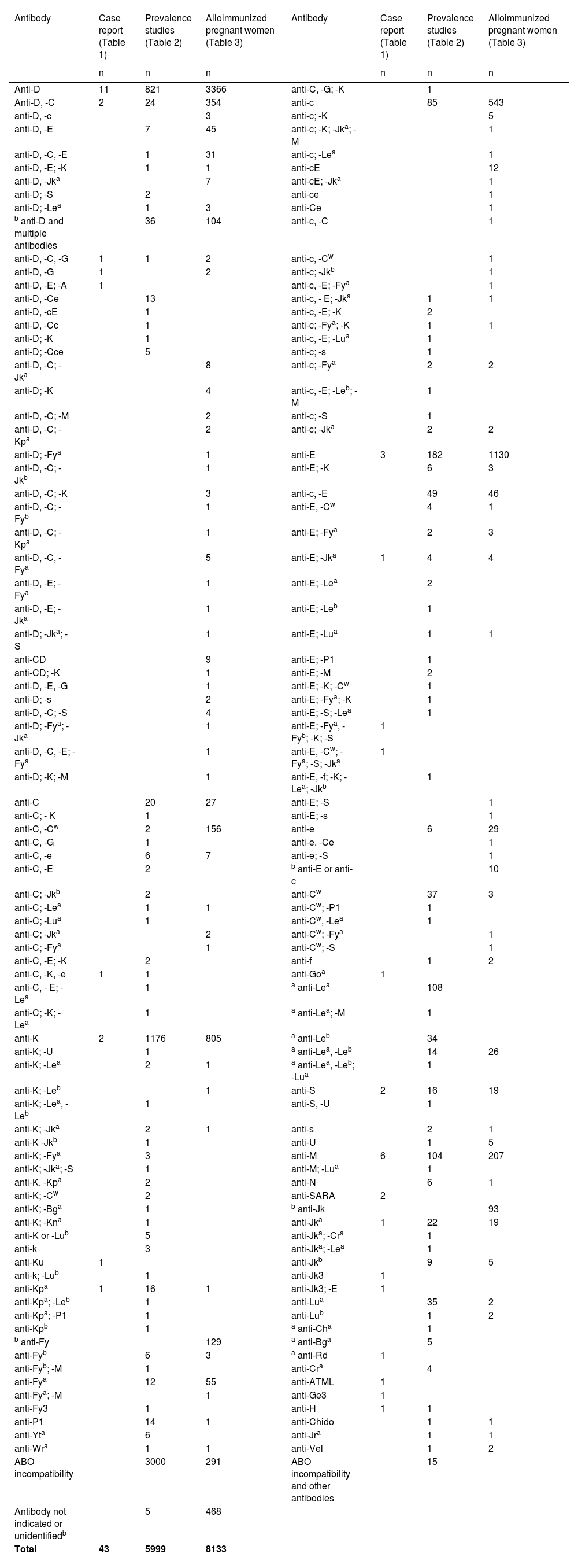
Details of the single or multiple maternal antibodies identified are reported in Table 4; multiple antibodies were found in 1791 cases. Nineteen different alloantibodies which cause HDFN were cited in case reports. ABO incompatibility was found in 3307 cases.
RBC antibodies present in maternal plasma.
| Antibody | Case report (Table 1) | Prevalence studies (Table 2) | Alloimmunized pregnant women (Table 3) | Antibody | Case report (Table 1) | Prevalence studies (Table 2) | Alloimmunized pregnant women (Table 3) |
|---|---|---|---|---|---|---|---|
| n | n | n | n | n | n | ||
| Anti-D | 11 | 821 | 3366 | anti-C, -G; -K | 1 | ||
| Anti-D, -C | 2 | 24 | 354 | anti-c | 85 | 543 | |
| anti-D, -c | 3 | anti-c; -K | 5 | ||||
| anti-D, -E | 7 | 45 | anti-c; -K; -Jka; -M | 1 | |||
| anti-D, -C, -E | 1 | 31 | anti-c; -Lea | 1 | |||
| anti-D, -E; -K | 1 | 1 | anti-cE | 12 | |||
| anti-D, -Jka | 7 | anti-cE; -Jka | 1 | ||||
| anti-D; -S | 2 | anti-ce | 1 | ||||
| anti-D; -Lea | 1 | 3 | anti-Ce | 1 | |||
| b anti-D and multiple antibodies | 36 | 104 | anti-c, -C | 1 | |||
| anti-D, -C, -G | 1 | 1 | 2 | anti-c, -Cw | 1 | ||
| anti-D, -G | 1 | 2 | anti-c; -Jkb | 1 | |||
| anti-D, -E; -A | 1 | anti-c, -E; -Fya | 1 | ||||
| anti-D, -Ce | 13 | anti-c, - E; -Jka | 1 | 1 | |||
| anti-D, -cE | 1 | anti-c, -E; -K | 2 | ||||
| anti-D, -Cc | 1 | anti-c; -Fya; -K | 1 | 1 | |||
| anti-D; -K | 1 | anti-c, -E; -Lua | 1 | ||||
| anti-D; -Cce | 5 | anti-c; -s | 1 | ||||
| anti-D, -C; -Jka | 8 | anti-c; -Fya | 2 | 2 | |||
| anti-D; -K | 4 | anti-c, -E; -Leb; -M | 1 | ||||
| anti-D, -C; -M | 2 | anti-c; -S | 1 | ||||
| anti-D, -C; -Kpa | 2 | anti-c; -Jka | 2 | 2 | |||
| anti-D; -Fya | 1 | anti-E | 3 | 182 | 1130 | ||
| anti-D, -C; -Jkb | 1 | anti-E; -K | 6 | 3 | |||
| anti-D, -C; -K | 3 | anti-c, -E | 49 | 46 | |||
| anti-D, -C; -Fyb | 1 | anti-E, -Cw | 4 | 1 | |||
| anti-D, -C; -Kpa | 1 | anti-E; -Fya | 2 | 3 | |||
| anti-D, -C, -Fya | 5 | anti-E; -Jka | 1 | 4 | 4 | ||
| anti-D, -E; -Fya | 1 | anti-E; -Lea | 2 | ||||
| anti-D, -E; -Jka | 1 | anti-E; -Leb | 1 | ||||
| anti-D; -Jka; -S | 1 | anti-E; -Lua | 1 | 1 | |||
| anti-CD | 9 | anti-E; -P1 | 1 | ||||
| anti-CD; -K | 1 | anti-E; -M | 2 | ||||
| anti-D, -E, -G | 1 | anti-E; -K; -Cw | 1 | ||||
| anti-D; -s | 2 | anti-E; -Fya; -K | 1 | ||||
| anti-D, -C; -S | 4 | anti-E; -S; -Lea | 1 | ||||
| anti-D; -Fya; -Jka | 1 | anti-E; -Fya, -Fyb; -K; -S | 1 | ||||
| anti-D, -C, -E; -Fya | 1 | anti-E, -Cw; -Fya; -S; -Jka | 1 | ||||
| anti-D; -K; -M | 1 | anti-E, -f; -K; -Lea; -Jkb | 1 | ||||
| anti-C | 20 | 27 | anti-E; -S | 1 | |||
| anti-C; - K | 1 | anti-E; -s | 1 | ||||
| anti-C, -Cw | 2 | 156 | anti-e | 6 | 29 | ||
| anti-C, -G | 1 | anti-e, -Ce | 1 | ||||
| anti-C, -e | 6 | 7 | anti-e; -S | 1 | |||
| anti-C, -E | 2 | b anti-E or anti-c | 10 | ||||
| anti-C; -Jkb | 2 | anti-Cw | 37 | 3 | |||
| anti-C; -Lea | 1 | 1 | anti-Cw; -P1 | 1 | |||
| anti-C; -Lua | 1 | anti-Cw, -Lea | 1 | ||||
| anti-C; -Jka | 2 | anti-Cw; -Fya | 1 | ||||
| anti-C; -Fya | 1 | anti-Cw; -S | 1 | ||||
| anti-C, -E; -K | 2 | anti-f | 1 | 2 | |||
| anti-C, -K, -e | 1 | 1 | anti-Goa | 1 | |||
| anti-C, - E; -Lea | 1 | a anti-Lea | 108 | ||||
| anti-C; -K; -Lea | 1 | a anti-Lea; -M | 1 | ||||
| anti-K | 2 | 1176 | 805 | a anti-Leb | 34 | ||
| anti-K; -U | 1 | a anti-Lea, -Leb | 14 | 26 | |||
| anti-K; -Lea | 2 | 1 | a anti-Lea, -Leb; -Lua | 1 | |||
| anti-K; -Leb | 1 | anti-S | 2 | 16 | 19 | ||
| anti-K; -Lea, -Leb | 1 | anti-S, -U | 1 | ||||
| anti-K; -Jka | 2 | 1 | anti-s | 2 | 1 | ||
| anti-K -Jkb | 1 | anti-U | 1 | 5 | |||
| anti-K; -Fya | 3 | anti-M | 6 | 104 | 207 | ||
| anti-K; -Jka; -S | 1 | anti-M; -Lua | 1 | ||||
| anti-K, -Kpa | 2 | anti-N | 6 | 1 | |||
| anti-K; -Cw | 2 | anti-SARA | 2 | ||||
| anti-K; -Bga | 1 | b anti-Jk | 93 | ||||
| anti-K; -Kna | 1 | anti-Jka | 1 | 22 | 19 | ||
| anti-K or -Lub | 5 | anti-Jka; -Cra | 1 | ||||
| anti-k | 3 | anti-Jka; -Lea | 1 | ||||
| anti-Ku | 1 | anti-Jkb | 9 | 5 | |||
| anti-k; -Lub | 1 | anti-Jk3 | 1 | ||||
| anti-Kpa | 1 | 16 | 1 | anti-Jk3; -E | 1 | ||
| anti-Kpa; -Leb | 1 | anti-Lua | 35 | 2 | |||
| anti-Kpa; -P1 | 1 | anti-Lub | 1 | 2 | |||
| anti-Kpb | 1 | a anti-Cha | 1 | ||||
| b anti-Fy | 129 | a anti-Bga | 5 | ||||
| anti-Fyb | 6 | 3 | a anti-Rd | 1 | |||
| anti-Fyb; -M | 1 | anti-Cra | 4 | ||||
| anti-Fya | 12 | 55 | anti-ATML | 1 | |||
| anti-Fya; -M | 1 | anti-Ge3 | 1 | ||||
| anti-Fy3 | 1 | anti-H | 1 | 1 | |||
| anti-P1 | 14 | 1 | anti-Chido | 1 | 1 | ||
| anti-Yta | 6 | anti-Jra | 1 | 1 | |||
| anti-Wra | 1 | 1 | anti-Vel | 1 | 2 | ||
| ABO incompatibility | 3000 | 291 | ABO incompatibility and other antibodies | 15 | |||
| Antibody not indicated or unidentifiedb | 5 | 468 | |||||
| Total | 43 | 5999 | 8133 |
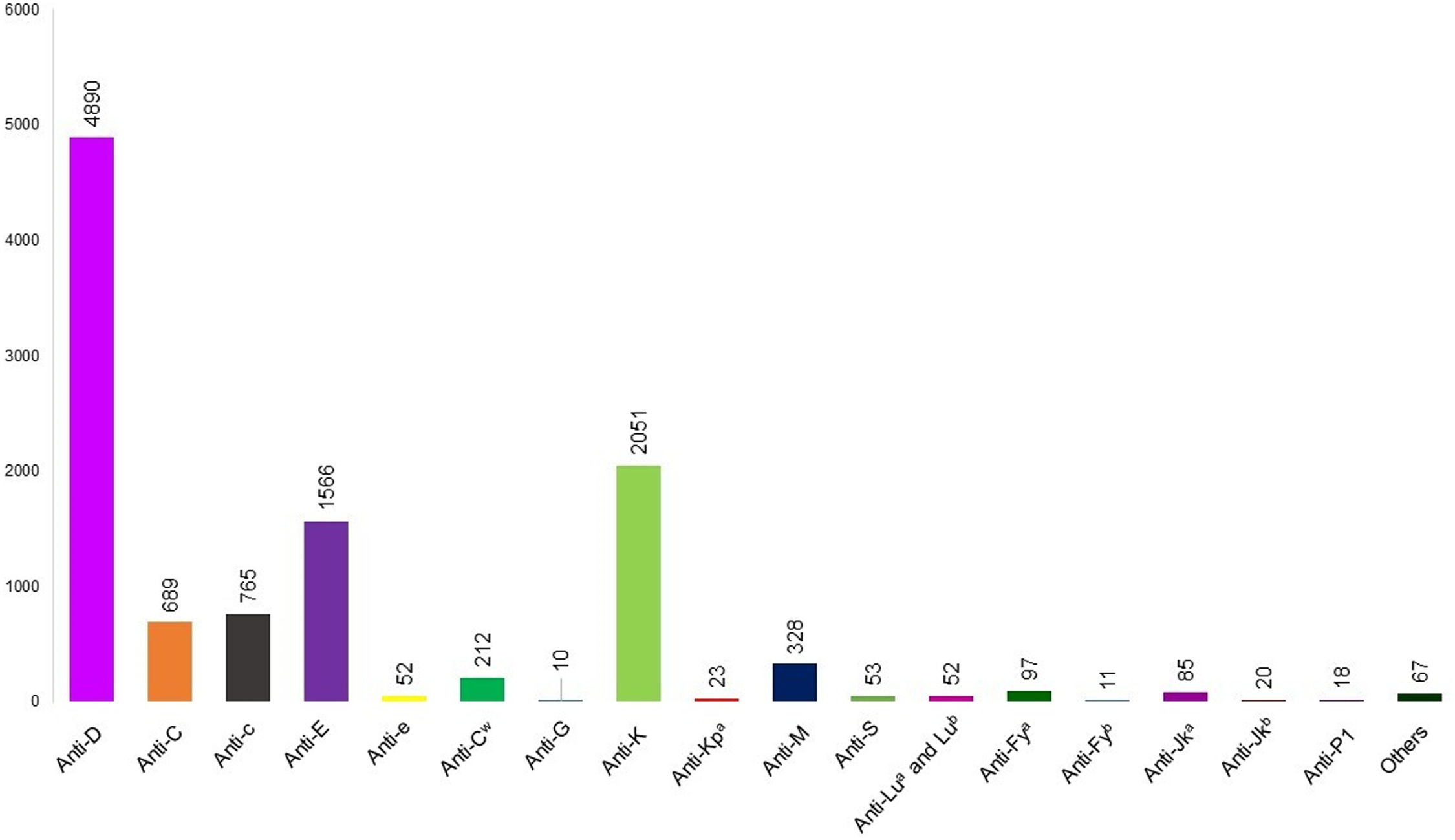
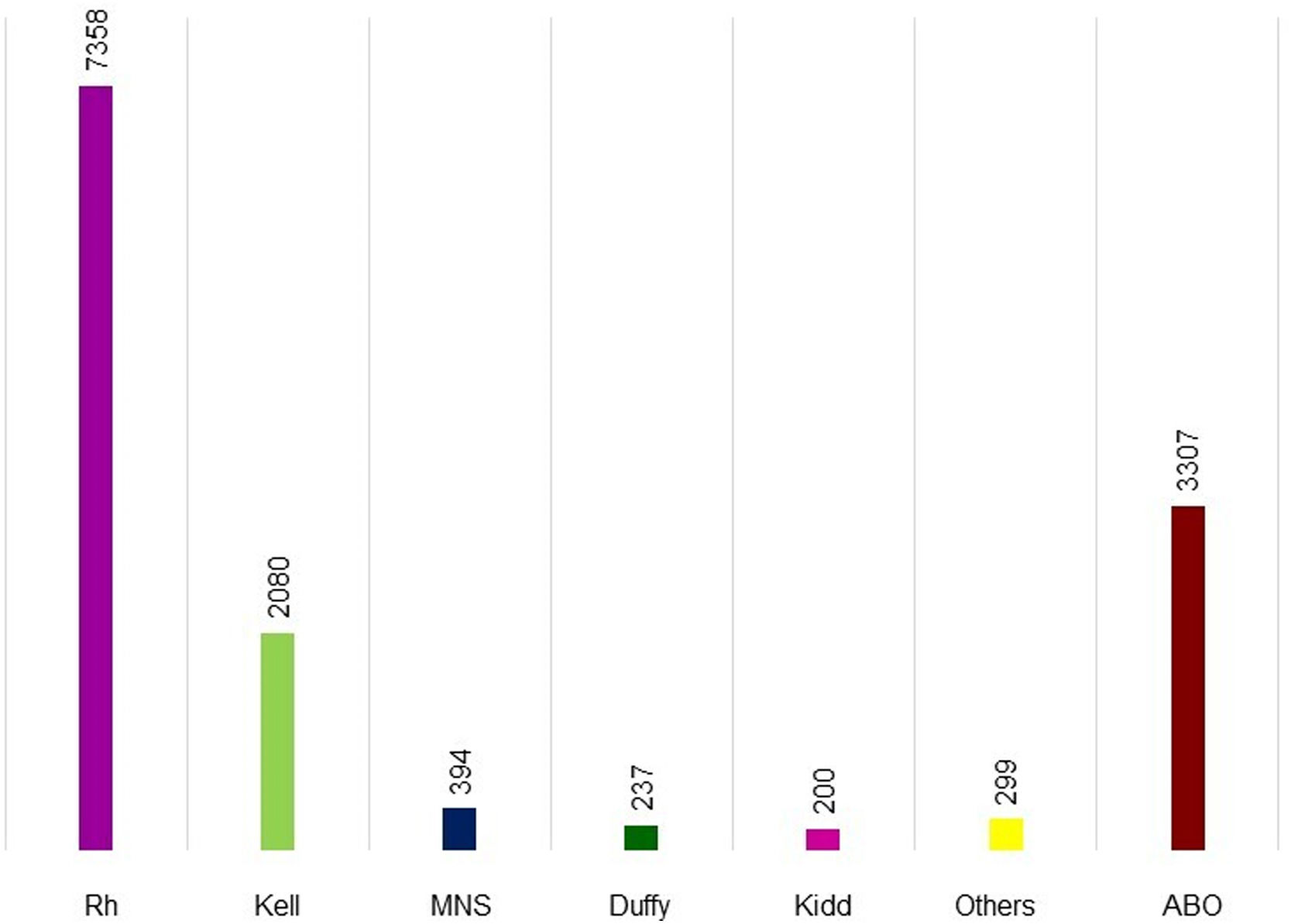
Figure 1 shows the alloantibodies found in isolation or combined with other antibodies. Anti-D (4890 cases), anti-K (2051 cases), and anti-E (1566 cases) were the most common in maternal plasma. Figure 2 shows the antibodies against RBC antigens classified according to the blood group system. Rh was the commonest with 7358 cases, followed by Kell (2080 cases), MNS (394 cases), Duffy (237 cases), and Kidd antibodies (200 cases). Anti-Lewis is not mentioned in Figures 1 or 2 because anti-Lewis antibodies are not implicated in HDFN.78
RBC antibodies present in maternal plasma. anti-N (n = 7), anti-U (n = 7), anti-Yta (n = 6), anti-Bga (n = 6), anti-s (n = 5), anti-Cra (n = 5), anti-f (n = 4), anti-k (n = 4), anti-Vel (n = 3), anti-Jk3 (n = 2), anti-SARA (n = 2), anti-H (n = 2), anti-Chido (n = 2), anti-Jra (n = 2), anti-Wra (n = 2), anti-Goa, anti-Kna, anti-Ku, anti-Kpb, anti-Cha, anti-Rd, anti-ATML, anti-Ge3 (n = 1 each).
HDFN is an important public health problem due to maternal-fetal incompatibility, which occurs due to fetal or neonatal hemolysis caused by IgG antibodies that cross the placenta.2 Just the presence of alloantibodies in maternal plasma is not sufficient to cause HDFN, it is necessary that antibodies cross the placenta and the fetus or neonate have the antigen expressed on their RBCs.
This review found 13,966 reports of alloimmunized pregnant women with anti-D being the most common alloantibody (35.01 %) followed by anti-K (14.69 %), anti-E (11.21 %), anti-c (5.48 %), anti-C (4.93 %) and anti-M (2.35 %). From prevalence studies, 0.17 % of pregnant women were alloimmunized, and from studies on alloimmunized pregnant women, 36.84 % had a baby with HDFN; these figures might be higher because some studies do not mention the clinical outcome of newborns. When verifying the geographical distribution of alloantibody studies, most were performed in Europe (35 studies), America (16 studies), and Asia (15 studies). These data show the need to perform more studies in other locations, thereby contributing to the understanding of alloimmunization and HDFN, for example, which antibody is the most involved in HDFN.
The Rh blood system is a highly polymorphic blood group79 with 56 antigens having been described.80 The most important antigens are D, E, e, C, c. Rh antibodies were found in the plasma of 7358 (52.69 %) of the 13,966 pregnant women, either in isolation or with other antibodies. Anti-E was the second most common alloantibody of the Rh system, followed by anti-c, anti-C, anti-Cw, anti-e, anti-G, anti-f and anti-Goa. The anti-G alloantibody is important for the correct identification of this latter alloantibody and differentiation from the anti-C and anti-D alloantibodies because if a pregnant woman does not present anti-D, prophylaxis is strongly recommended to avoid RhD alloimmunization.18,28
ABO maternal-fetal incompatibility is common because ABO antibodies develop naturally. Matteocci et al.46 analyzed HDFN related to ABO incompatibility: 81 babies had a positive direct antiglobulin test (DAT) and 32 required invasive treatments (exchange transfusions or intravenous immunoglobulins).46 When ABO incompatibility was present, the O blood group was associated with reduced alloimmunization compared to other blood group antigens because of the presence of anti-A/anti-B antibodies in maternal plasma. This could occur because of the clearance of A or B fetal RBCs in maternal plasma mediated by ABO antibodies. This clearance might avoid other alloimmunizations. Doyle et al. analyzed anti-D levels and IUT; on comparing ABO blood groups in respect to IUT, women of the A blood group have a higher risk of carrying a fetus with significant HDFN compared to women of the O blood group.52
Kell alloantibodies are the second most commonly related to HDFN.44 Anti-K is associated with a risk of HDFN, nonetheless the titer does not necessarily correlate with the clinical severity of HDFN.26 In a retrospective cohort that evaluated 1026 Kell immunized pregnancies, a cut-off value for risk of severe HDFN was established with 93 K-positive fetuses; the value of 4 identified a risk of severe HDFN.44 However, severe cases of HDFN occur with lower anti-K titers, demonstrating that there is no correlation between clinical outcome and the titer.81 Accordingly, any case of an anti-K positive pregnancy should be accompanied when it is not possible to predict the fetus phenotype because anti-K antibodies cause erythropoiesis suppression and the destruction of erythroid progenitors.
Anti-M is one case of alloantibodies in which IgM and IgG antibodies are found. IgG antibodies can cross the placenta and cause HDFN to variable degrees. A review of the Japanese population presented 33 cases of HDFN caused by anti-M; of those 29 developed severe HDFN, five presented IgG subclasses (IgG1 or IgG3).11 Six case reports related to anti-M antibodies describe babies with HDFN, three with different titers and clinical outcomes that required transfusions (RBCs and platelets), one required intrauterine transfusion for severe fetal anemia, and two evolved to intrauterine death. These data show that the severity was independent of the anti-M titer and the importance of the differentiation of IgG and IgM antibody classes.11,13,21
The physiopathology of anti-M is similar to those of anti-K and anti-Gerbich type 3 (anti-Ge3) with mechanisms of apoptotic or erythropoietic suppression (extracellular hemolysis) differing from the physiopathology of anti-D.82
Kidd blood group antigens are implicated in hemolytic transfusion reactions and HDFN. The most common antibodies are anti-Jka and anti-Jkb, which cause mild to severe disease. There is another antibody, anti-Jk3, which can be induced by alloimmunization in Kidd null phenotype people.24 Anti-Jk3 reacts with both Jka and Jkb antigens.24 It remains unclear which anti-Jk3 titers produce a clinically significant risk of HDFN; according to Lawicki et al.,24 fetuses with titers of 16 or higher should be monitored.
Multiple maternal alloantibodies could represent an increased risk of developing HDFN. One study from Israel showed that 6.8 % of pregnancies with multiple alloantibodies develop severe HDFN.3 Phung et al. compared the mean estimated daily decrease in Hb between the first and second IUT when the pregnant woman had anti-D in isolation and when it was associated with two other antibodies. This study showed that the drop was lower when the pregnant had only anti-D.58 These datasets suggest that the presence of multiple maternal alloantibodies could point toward an increase in the risk of the severity of HDFN. In this review, Table 4 presents observation studies and six case reports in which the HDFN was caused by multiple alloantibodies.6,7,16–18,24,37,38,40,43,58
One key factor in the risk of HDFN is the antibody class; IgM antibodies are not implicated in HDFN because they are not capable of crossing the placenta however IgG antibodies cross the placenta and can cause HDFN. IgG antibodies are subclassified into IgG1, IgG2, IgG3, and IgG4. The severity of HDFN may be related to the IgG subclasses; IgG1 and IgG3 cause severe HDFN and so these parameters should be included in protocols for measuring the intensity of the HDFN.40
When all antigen sites are occupied by the respective antibody, the reaction of these RBCs with commercial antisera produce a false negative phenotype in the antihuman globulin phase.26 These cases are rare but can occur when a direct antiglobulin test is positive. Using methods without the antihuman globulin phase with saline monoclonal IgM antiserum is an option to solve these cases, but these reagents are not always available.83 An IgG blocking technique can be used for RBCs that have a weak to moderately positive (2+) DAT;84 other methods use ethylenediaminetetraacetic acid (EDTA) elution, chloroquine diphosphate (CPD) and heat elution to remove IgG antibodies from RBCs.26
Molecular tests present an option to solve cases of false negative phenotypes in newborns. Novoselac et al. used a genotyping test (single specific primer-polymerase chain reaction - PCR-SSP) to confirm the K*01/K*02 genotype in a baby and solve an anti-K mediated HDFN.26 Lawicki et al. also used this test to confirm the JK*A/JK*B genotype in a baby and solve an anti-Jk3 mediated HDFN.24
Knowing the molecular basis of blood group antigens is also important to predict phenotypes and the formation of null alleles that might cause a lack of expression of the phenotype. The ISBT lists all blood group antigens and genes involved in antigen expressions. The null phenotype is caused by different mutations in the genes; some have higher frequencies in specific population groups, for example, the Fy null allele (c.1-67T>C, rs2814778) in African descendants85 and the Jk null allele (T871C mutation) in Polynesian descendants.86
Sequencing tests are useful to understand unexplained discrepancies between phenotype and genotype, discover antigen variants and predict the risk and significance of alloimmunization to cause HDFN and hemolytic transfusion reactions.25 In order to understand the risk of HDFN, it is recommended to perform maternal and paternal phenotyping or genotyping to predict fetal phenotype.66 However, paternal phenotyping is not always available in cases of suspected HDFN.24
The screening of irregular RBC antibodies and maternal antibody titers using the indirect antiglobulin test during prenatal care is important for the correct diagnosis and early clinical intervention as is using Doppler ultrasound to measure the peak systolic blood flow velocity in the middle cerebral artery.65 The correct identification of maternal alloantibodies and estimated risk of the fetus carrying the RBC antigen is important in prenatal exams.77 The precise identification of one or multiple alloantibodies and their clinical significance help to select packed RBC units for transfusion when IUT are needed, and after birth if the newborn needs a transfusion. In the newborn, it is important to perform DAT, and when the DAT is positive, to perform elution to identify the antibody bound to the RBC membrane.78
Some developed countries use non-invasive prenatal testing with cell-free fetal DNA (cffDNA) circulating in the maternal plasma of pregnant women to identify the fetus genotype and estimate the risk of HDFN.87 RhD incompatibility using cffDNA is an efficient prevention strategy as anti-D prophylaxis will only be provided if the fetus is RhD-positive.61 cffDNA is useful for other RBC antigens to determine if the fetus presents antigens for which the mother has alloantibodies and determine the risk of HDFN. This method is non-invasive but it is not available in all hemotherapy services.
IUTs were needed at least once in 1938 fetuses in this review however, this data is limited because some studies did not mention fetal outcomes. IUT is an intervention related to the severity of anemia, and early IUT (before 20 weeks) is associated with a higher risk of fetal injury.73 Despite the risk, IUT is an important intervention to treat fetal anemia. The need for IUT should be considered in prenatal care. However, there are great differences in the prenatal care provided in different countries and some pregnant women who need an IUT do not receive it.
Unfortunately, some countries do not screen all pregnant women for alloantibodies, due to many reasons such as the absence of universal healthcare, failure to recognize events during pregnancy that could trigger alloimmunization, and geographical difficulties in accessing a hemotherapy reference center.77
ConclusionInvesting in early diagnosis is important for the management of risks and complications related to the development of HDFN. Approaches using serological and molecular tests are useful for early diagnosis. Knowing the physiopathology of alloantibodies is also helpful in understanding the evolution of HDFN. Furthermore, considering the high diversity of blood group alleles, there is a need to study the frequency of pregnant alloimmunization and HDFN in different populations.
Authors’ contributionsMMOR and MF designed the study, performed the systematic literature search, performed data interpretation, and wrote the manuscript; MMOR, DM, SA and MF analyzed the data. All authors were involved in writing the paper and approved the final version.
We thank FAPERGS (PqG 02/2014, 1412373-4 PQG) for their financial support.