This study investigated the occurrence of the p190 and p210 breakpoint cluster region-Abelson (BCR-ABL) rearrangements in adults with acute lymphoblastic leukemia and possible associations with clinical and laboratory characteristics and survival.
MethodsForty-one over 18-year-old patients with acute lymphoblastic leukemia of both genders followed-up between January 2008 and May 2012 were included in this study. Clinical and laboratory data were obtained from the medical charts of the patients. Reverse transcription polymerase chain reaction (RT-PCR) using specific primers was employed to identify molecular rearrangements.
ResultsAt diagnosis, the median age was 33 years, and there was a predominance of males (61%). The most common immunophenotype was B lineage (76%). BCR-ABL rearrangements was detected in 14 (34%) patients with the following distribution: p190 (28%), p210 (50%) and double positive (22%). Overall survival of patients with a mean/median of 331/246 days of follow up was 39%, respectively, negative BCR-ABL (44%) and positive BCR-ABL (28%).
ConclusionThese results confirm the high frequency of BCR-ABL rearrangements and the low survival rate of adult Brazilian patients with acute lymphoblastic leukemia.
Acute lymphoblastic leukemia (ALL) in adults comprises a group of diseases with biological, clinical, laboratorial and prognostic heterogeneity characterized by abnormal proliferation and accumulation of immature lymphoid cells in the bone marrow and lymphoid tissues.1 Unlike ALL in children, the advances in the therapy in adults have been slow, with a mean survival of 35% in patients aged between 18 and 60 years.2 As a result, considerable effort has been made to identify markers that can be translated to the clinic as new prognostic tools and therapeutic targets.3
At present, the diagnosis and classification of acute leukemia depend on cytomorphologic, immunophenotypic, cytogenetic and molecular analyses. Molecular tests are part of the criteria for the risk classification system of the World Health Organization (WHO) and are used to evaluate the prognosis correctly and define therapeutic strategies.4
Of the various genetic alterations observed in adult ALL, the breakpoint cluster region-Abelson (BCR-ABL) fusion gene is the most common and is associated with a particularly poor prognosis.1,5,6 This gene rearrangement can present two distinct isoforms, p190 and p210 due to different breakpoints.7 Recent studies indicate that these two isoforms may be associated with different clinical phenotypes in adult ALL patients.8
The aim of this study was to investigate the occurrence of the p190 and p210 BCR-ABL rearrangements in adult ALL patients and to investigate possible associations with clinical and laboratory features and survival.
MethodsThe study group comprised 41 over 18-year-old patients of both genders diagnosed with ALL at the Fundação de Hematologia e Hemoterapia de Pernambuco (Hemope) from January 2008 to May 2012. The diagnosis was established by clinical, cytomorphological and immunophenotypic criteria. The standard treatment protocol used was the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (HyperCVAD) regimen.9 This project was approved by the Research Ethics Committee of the institution (#17/2010) and the study was conducted in accordance with the Declaration of Helsinki 2008.
Clinical and laboratory data were obtained from the patients’ records. Samples of peripheral blood and bone marrow were collected after informed consent had been given. The identification of the p190 and p210 BCR-ABL gene rearrangements was performed by reverse transcription polymerase chain reaction (RT-PCR) according to the international BIOMED-1 protocol.10 The following controls were used in the RT-PCR reactions: positive, negative, endogenous and contamination.
Statistical analysis was performed using the Bioestat 5.0 and Stata 9.1 programs. The t-test was used to compare the groups regarding age, leukocyte count, blasts, platelet count and hemoglobin. The Fisher exact test was used for the categorical variables (gender and immunophenotype). Overall survival was calculated using the Kaplan–Meier Log rank method. p-values <0.05 were considered statistically significant.
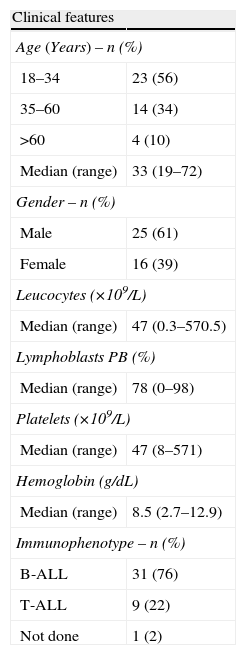
ResultsOf the 41 patients analyzed, ALL was more prevalent in young adults and men and the most common immunophenotype was B lineage (Table 1).
Clinical features at diagnosis of 41 adult patients with acute lymphoblastic leukemia from 2008 to 2012 at HEMOPE.
| Clinical features | |
| Age (Years) – n (%) | |
| 18–34 | 23 (56) |
| 35–60 | 14 (34) |
| >60 | 4 (10) |
| Median (range) | 33 (19–72) |
| Gender – n (%) | |
| Male | 25 (61) |
| Female | 16 (39) |
| Leucocytes (×109/L) | |
| Median (range) | 47 (0.3–570.5) |
| Lymphoblasts PB (%) | |
| Median (range) | 78 (0–98) |
| Platelets (×109/L) | |
| Median (range) | 47 (8–571) |
| Hemoglobin (g/dL) | |
| Median (range) | 8.5 (2.7–12.9) |
| Immunophenotype – n (%) | |
| B-ALL | 31 (76) |
| T-ALL | 9 (22) |
| Not done | 1 (2) |
No statistically significant differences were found between the groups of BCR-ABL positive and negative patients in respect to the clinical and laboratory variables. However, the p210 BCR-ABL patients had higher leukocyte counts and all p190 BCR-ABL patients had the B immunophenotype (Table 2).
Summary of clinical and laboratory parameters of 41 adult patients diagnosed with acute lymphoblastic leukemia at HEMOPE.
| Variable | BCR-ABL−(n=27) | BCR-ABL+(n=14) | BCR-ABL+p190(n=4) | BCR-ABL+p210(n=7) | BCR-ABL+p190/p210(n=3) |
| Age (years) | |||||
| Median (range) | 35 (19–72) | 38 (20–72) | 45 (22–61) | 35 (20–72) | 34 (20–48) |
| Leucocytes (×109/L) | |||||
| Median (range) | 30.4 (1–492) | 77.1 (0.3–570) | 76.6 (0.3–378) | 96.0 (6.7–570) | 7.7 (4.2–298.8) |
| Platelets (×109/L) | |||||
| Median (range) | 41 (8–571) | 69 (18–213) | 175.5 (18–213) | 60 (27–155) | 70 (60–112) |
| Lymphoblasts (%) | |||||
| Median (range) | 78 (0–97) | 80 (0–98) | 75 (0–93) | 85 (31–98) | 36 (3–94) |
| Immunophenotypea– n (%) | |||||
| B-ALL | 21 (78.0) | 10 (71.5) | 4 (100.0) | 3 (43.0) | 3 (100.0) |
| T-ALL | 5 (22.0) | 4 (28.5) | 0 (0) | 4 (57.0) | 0 (0) |
−: negative; +: positive.
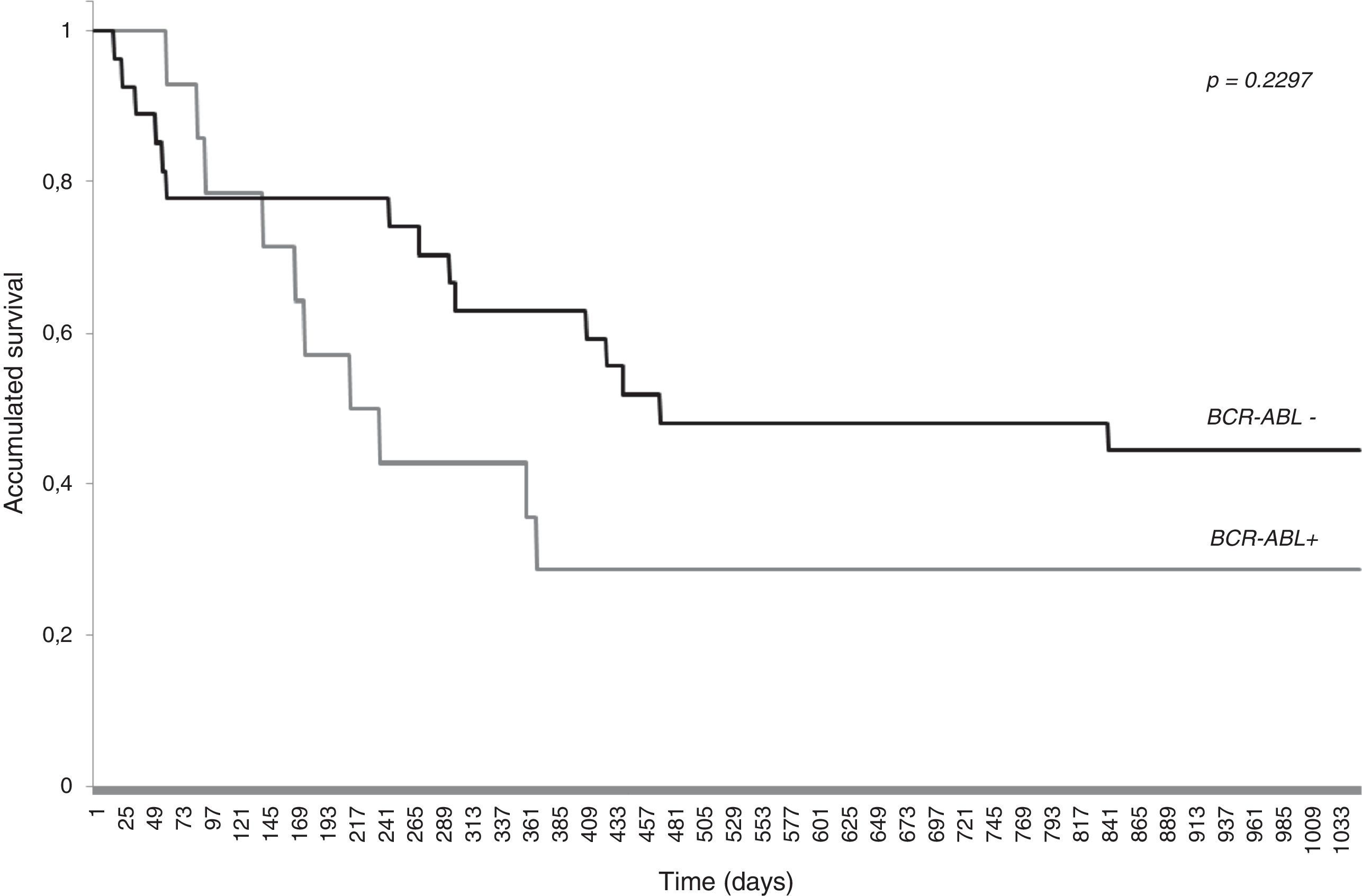
The overall survival was 39% with a mean follow-up of 331 days (median 246 days). Survival was lower for BCR-ABL positive (28%) than for BCR-ABL negative (44%) patients. The Log-rank test, however, showed no statistically significant difference (p-value=0.2297) between the survival curves of the two groups (Fig. 1). The mortality rate of BCR-ABL positive patients is 1.94 times greater [95% Confidence Interval (CI): 0.80–4.26] than the BCR-ABL negative individuals, but again the difference is not statistically significant (p-value=0.148).
DiscussionThe median age of the patients was 33 years, which is similar to several published series.11–17 Males predominated in the sample, which is in accordance with the main multicenter studies.11–15,18–21 The results of several studies have shown similar numbers of leucocytes11,12,20 at diagnosis, including the percentage of blasts in the peripheral blood13 and platelet count.17 The 76% frequency of B cell phenotype is in accordance with various published studies.11,12,17,21–23
The 34% frequency of the BCR-ABL rearrangement is similar to that found in several studies with values ranging from 17% to 37%,1,11–14,17–22 including in elderly patients, as reported by Larson.24 No published Brazilian studies with data regarding the molecular analysis of BCR-ABL in adult ALL patients were found for comparison. A case series of 42 adult Brazilian patients showed 7% of Ph+ samples.25
The results presented in this study confirm the high frequency of BCR-ABL rearrangements in adult ALL patients, but, differ from other studies regarding the type of isoforms found. Gleiβer et al.21 showed a 37% positivity for the BCR-ABL fusion gene in 478 adult ALL patients including the p190 (77%) and p210 (20%) rearrangements and both isoforms (3%). Dombret et al.22 found the following frequencies among 154 adult ALL patients: p190 (68%), p210 (28%) and both isoforms (4%). The explanation for our results is not clear, including the occurrence of BCR-ABL positivity in T-ALL cases19, but may be due to sample size or characteristics of the population studied, as well as patients with chronic myeloid leukemia in acute phase.26
The analysis of the survival curves, in addition to confirming the low rate of overall survival for adult patients diagnosed with ALL,9,14,20,21,23 also suggests an increased adverse prognosis conferred by the presence of BCR-ABL rearrangements,2,18,21–23,27 and therefore a need for other therapeutic modalities, including targeted therapies and bone marrow transplantation.28 The range of the confidence interval of the mortality rate suggests that the sample size was too small to show a difference and that increasing it would make it more evident. Phenotypic differences between p190 and p210 BCR-ABL patients is controversial.8,21 Further studies with a larger sample size, including elderly patients, are needed to better characterize the association between these rearrangements and different phenotypic expressions and survival. The detection of the BCR-ABL fusion gene is important for the classification of risk groups of ALL patients and the correct targeting of therapy.2,3 Moreover, in addition to the BCR-ABL fusion gene, other rearrangements, such as E2A-PBX1, TEL-AML1, MLL-AF4, should be screened, because they also have prognostic significance.29
ConclusionOur results provide the first published evidence of the high frequency of BCR-ABL and poor survival in adult Brazilian ALL patients. The study confirms the importance of detecting BCR-ABL rearrangements for the treatment and prognosis of these patients.
Authors’ contributions and declaration of conflicts of interestThe authors Ilana de França Azevedo and Rui Milton Patrício da Silva Júnior contributed equally to the development of the work. The authors declare no conflicts of interest.
The authors thank the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (#APQ-1343-4.00-08) for funding the study and Prof. Ricardo de Alencar Ximenes Arraes for his support with the statistics.