The emergence of oligoclonal bands, proteins differing from those originally identified at diagnosis, has been reported in multiple myeloma patients after high-dose chemotherapy followed by autologous stem cell transplantation and after successful conventional chemotherapy. The clinical relevance of oligoclonal bands remains unclear, but their emergence has been associated with better prognosis. The aim of the present study was to determine the prevalence, clinical characteristics and prognostic impact of the presence of oligoclonal bands in multiple myeloma patients.
MethodsA retrospective cohort study was conducted. The study included newly diagnosed multiple myeloma patients with at least very good partial response after conventional dose or high-dose chemotherapy followed by autologous stem cell transplantation. The emergence of oligoclonal bands was identified using serum protein electrophoresis as well as serum and urine immunofixation techniques.
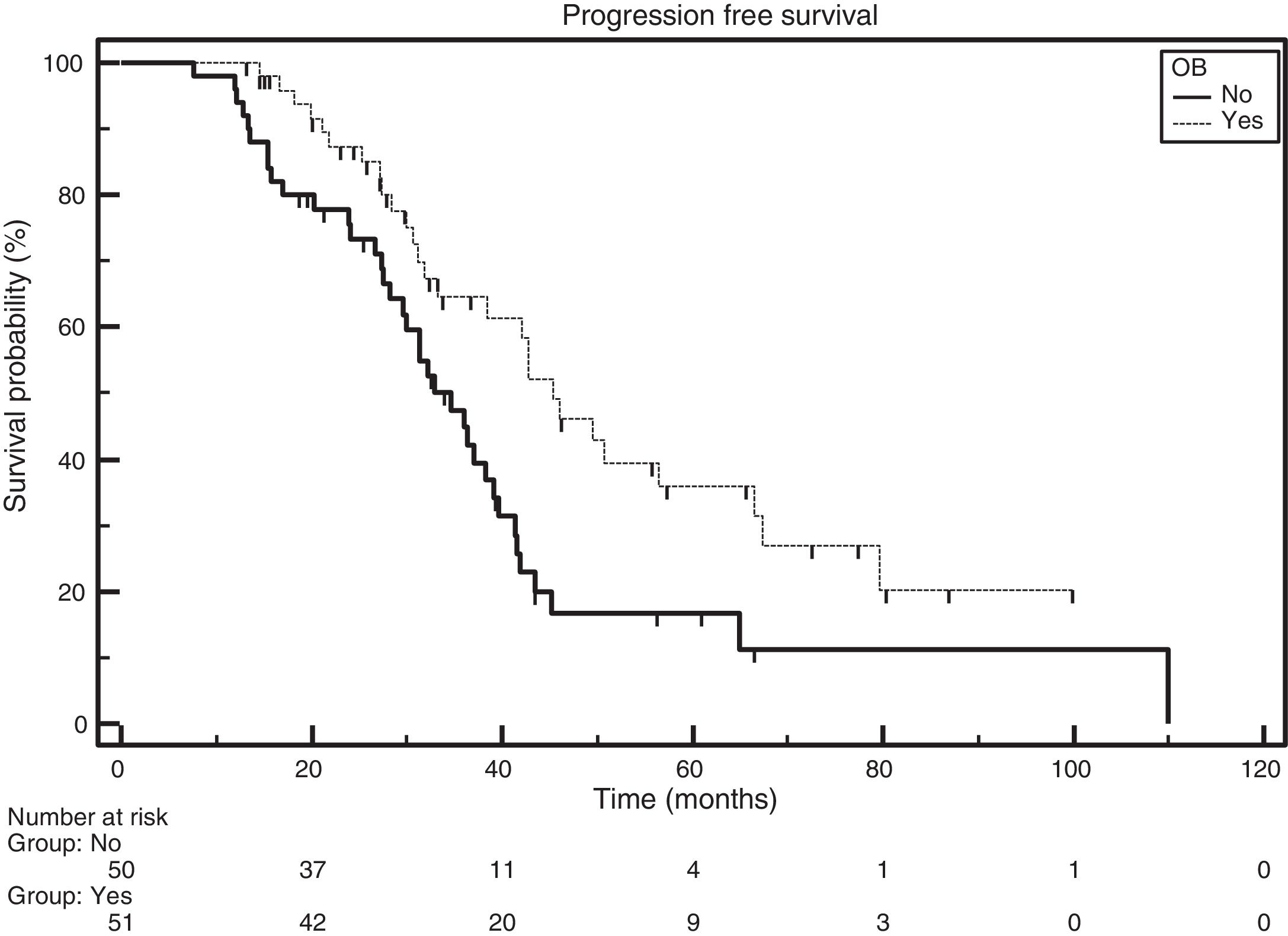
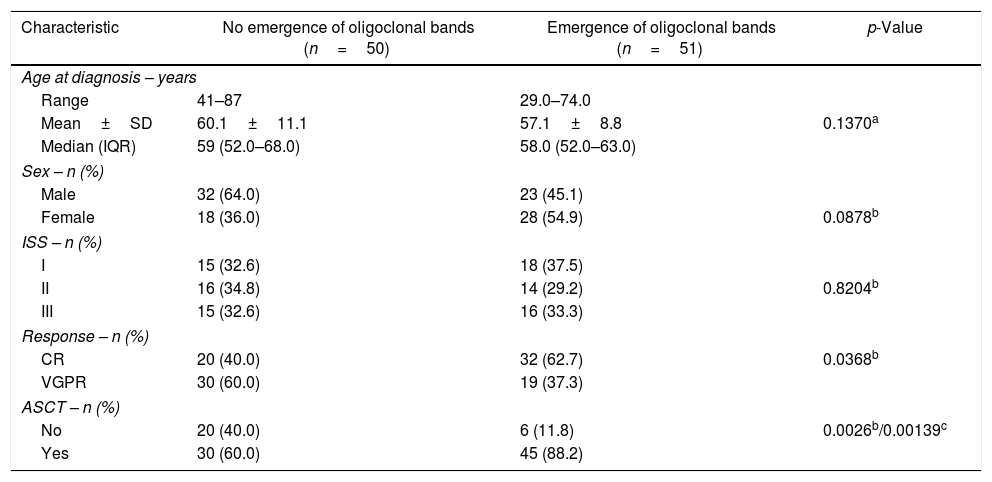
ResultsA total of 101 patients were included with a median follow-up of 42 months. In total, 55% were male, and the median age was 58 years (29–87 years). Fifty-one (50.5%) patients developed oligoclonal bands. They comprised 60% (45/75) of patients treated with autologous stem cell transplantation and 23% (6/26) of those who were not transplanted. Patients with oligoclonal bands showed better progression-free survival than those without the emergence of oligoclonal bands (p-value=0.0075).
ConclusionThe prevalence of oligoclonal bands in this study population was 50.5% with its frequency being greater in cases treated with autologous stem cell transplantation and in those attaining complete remission. Complete remission was more important than the emergence of oligoclonal bands on progression-free survival.
Multiple myeloma (MM) is a hematologic disorder that is characterized by the clonal expansion of plasma cells in the bone marrow. These plasma cells are responsible for the production of a unique monoclonal immunoglobulin with a constant isotype and light chain restriction that can be found in serum and/or urine and is termed paraprotein or the M component.1 The measurement of this monoclonal protein by serum protein electrophoresis (SPE) and immunofixation is invaluable for monitoring patients with MM.1
Since the introduction of high-dose melphalan followed by rescue using autologous hematopoietic stem cell transplantation (ASCT), the emergence of oligoclonality or oligoclonal bands (OB) has been described in numerous studies.2–9 This is defined as follows: (1) a new monoclonal component identified by SPE, (2) a variation of the immunoglobulin subtype, and/or (3) the emergence of more than one immunoglobulin subtype detected by serum or urine immunofixation that differs from the initial pattern observed at diagnosis.2–5 These invariably small components can remain undetected by SPE but are identified by immunofixation in up to 66% of cases.3
The prevalence of OB varies, ranging from 6.6% to 73%.3,6 They are commonly found in patients who have undergone ASCT.2,3,7,8 However, OB can also emerge after conventional chemotherapy, particularly following the use of the novel agents, such as immunomodulators (IMIDs) and proteasome inhibitors (PI).4,5 The presence of OB has been associated with superior response rates.2,5 While initially described as a mere transient phenomenon of immunologic recovery, some authors have suggested that the appearance of these bands is associated with improved prognosis and longer survival.2–4,9,10
The emergence of OB can be mistaken as disease progression, leading to unwarranted changes in treatment. Thus, to better understand the frequency, the clinical characteristics and the prognostic impact of OB, the clinical records and the results of SPE and immunofixation tests of MM patients who had at least very good partial response (VGPR) after treatment were analyzed. This is the first study analyzing the impact of OB in MM patients treated in Brazil.
MethodsThe medical records of 328 patients who were treated at two referral centers for MM in the Brazilian national health system (Santa casa de São Paulo Hospital and University Hospital of the Universidade Federal da Bahia) from July 2003 to June 2013 were reviewed. Participants had achieved at least VGPR, defined as serum and urine M-protein detectable by immunofixation, but not by SPE, or who achieved ≥90% reduction in serum M-protein plus urine M-protein level <100mg/24h after first-line therapy,11 specifically, conventional doses of chemotherapy or high-dose chemotherapy and ASCT. A total of 101 patients were included for the identification of the emergence of OB using the SPE and immunofixation techniques. The study was approved by the Research Ethics Committees of both institutions.
The analyzed variables were sex and age at diagnosis, type of immunoglobulin secreted, Eastern cooperative oncology group (ECOG) performance status, staging systems (Durie-Salmon and International staging system – ISS), treatment (high-dose chemotherapy and ASCT or conventional chemotherapy), response assessment and emergence of OB.
The emergence of OB was defined as (1) new monoclonal spike on SPE, which differed from the initial pattern evidenced by direct comparison of assays, (2) immunoglobulin subtype switching, and/or (3) more than one immunoglobulin subtype at serum and urine immunofixation.
The response criteria were based on the 2006 International myeloma working group.11 Overall survival was calculated from the start of treatment to death or loss to follow-up and progression-free survival (PFS) was calculated from the start of treatment to progression, death or loss to follow-up.
Categorical variables were compared using the Chi-square or Fisher exact tests, the t-test was used to compare age among groups and the Kaplan–Meier curve was employed for survival analysis, with comparison across groups using the log rank test. Kaplan–Meier analyses were also used to identify potential predictor variables for PFS. These variables were included in the Cox regression model that also had the treatment group as a covariate. Statistical analyses were performed using MedCalc software (Mariakerke, Belgium, v 11.3.3.0).
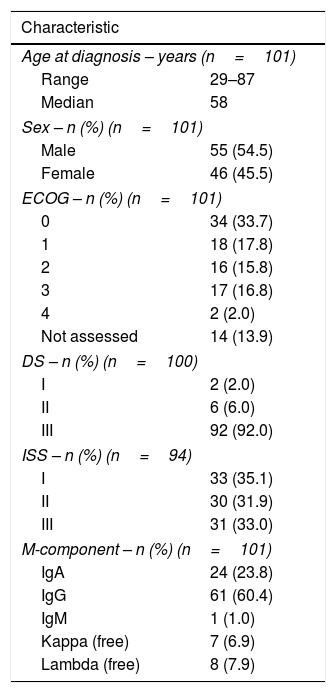
ResultsA total of 101 multiple myeloma patients with VGPR or better responses after conventional or high-dose chemotherapy as the first line of treatment were included. The median follow-up was 42 months (range: 7.63–131.4 months). Fifty-five (54.5%) patients were male and 46 (45.5%) were female. The median age at diagnosis was 58 (range: 29–87) years. The clinical characteristics of the study population are given in Table 1.
Clinical and laboratory characteristics of the study population.
| Characteristic | |
|---|---|
| Age at diagnosis – years (n=101) | |
| Range | 29–87 |
| Median | 58 |
| Sex – n (%) (n=101) | |
| Male | 55 (54.5) |
| Female | 46 (45.5) |
| ECOG – n (%) (n=101) | |
| 0 | 34 (33.7) |
| 1 | 18 (17.8) |
| 2 | 16 (15.8) |
| 3 | 17 (16.8) |
| 4 | 2 (2.0) |
| Not assessed | 14 (13.9) |
| DS – n (%) (n=100) | |
| I | 2 (2.0) |
| II | 6 (6.0) |
| III | 92 (92.0) |
| ISS – n (%) (n=94) | |
| I | 33 (35.1) |
| II | 30 (31.9) |
| III | 31 (33.0) |
| M-component – n (%) (n=101) | |
| IgA | 24 (23.8) |
| IgG | 61 (60.4) |
| IgM | 1 (1.0) |
| Kappa (free) | 7 (6.9) |
| Lambda (free) | 8 (7.9) |
ECOG: Eastern cooperative oncology group; DS: Durie-Salmon; ISS: International staging system; M-component: monoclonal component.
Seventy-five patients were considered eligible for ASCT. For induction therapy, the cyclophosphamide, thalidomide and dexamethasone regimen (CTD), used in 52% of patients, was the most common treatment. This was followed by thalidomide and dexamethasone (TD) in 21.3% of patients and by vincristine, doxorubicin and dexamethasone (VAD) in 17.3% of patients. The patients who were considered ineligible for ASCT (n=26) were mainly treated with CTD (38.5%), melphalan and prednisone (23%), and melphalan, prednisone and thalidomide (MPT) (15.4%).
OB emerged in 51 (50.5%) patients, of which 54.9% were female and 45.1% were male. Despite a slight predominance of females, there was no statistically significant difference between the groups. The median age of these patients was 58 (range: 52–63) years. From the 51 cases in which OB emerged, 45 cases (88.2%) were ASCT patients, and six (11.8%) were ineligible cases. An analysis of the prevalence of OB by treatment type revealed that 60% of ASCT patients presented OB versus only 23% of transplant-ineligible patients. Achieving complete response (CR) was associated with a greater likelihood of the emergence of OB, and specifically, 62.7% of CR patients developed OB (p-value=0.03). Table 2 shows the comparison of patients with and without the emergence of OB.
Comparison of patients with and without the emergence of oligoclonal bands.
| Characteristic | No emergence of oligoclonal bands (n=50) | Emergence of oligoclonal bands (n=51) | p-Value |
|---|---|---|---|
| Age at diagnosis – years | |||
| Range | 41–87 | 29.0–74.0 | |
| Mean±SD | 60.1±11.1 | 57.1±8.8 | 0.1370a |
| Median (IQR) | 59 (52.0–68.0) | 58.0 (52.0–63.0) | |
| Sex – n (%) | |||
| Male | 32 (64.0) | 23 (45.1) | |
| Female | 18 (36.0) | 28 (54.9) | 0.0878b |
| ISS – n (%) | |||
| I | 15 (32.6) | 18 (37.5) | |
| II | 16 (34.8) | 14 (29.2) | 0.8204b |
| III | 15 (32.6) | 16 (33.3) | |
| Response – n (%) | |||
| CR | 20 (40.0) | 32 (62.7) | 0.0368b |
| VGPR | 30 (60.0) | 19 (37.3) | |
| ASCT – n (%) | |||
| No | 20 (40.0) | 6 (11.8) | 0.0026b/0.00139c |
| Yes | 30 (60.0) | 45 (88.2) | |
SD: standard deviation; IQR: interquartile range; ISS: International staging system; CR: complete response; VGPR: very good partial response; ASCT: autologous hematopoietic stem cell transplantation.
OB were detectable by serum or urine immunofixation, but not by SPE, in 64% of cases. The most frequent finding was the combination of more than one immunoglobulin subtype (IgG, kappa and Lambda), which was identified in 21 cases.
The median PFS for the entire group was 38.4 months. The median PFS were 45.4 and 34.7 months for patients with and without the emergence of OB, respectively [Hazard ratio (HR)=0.5249; 95% confidence interval (CI): 0.3194–0.8625; p-value=0.0075; Figure 1].
The median overall survival for the entire group was 89.9 months. Patients without the emergence of OB had a median overall survival of 80.7 months versus 89.9 months for patients with the emergence of OB (HR: 0.5291; 95% CI: 0.2293–1.2212; p-value=0.1431).
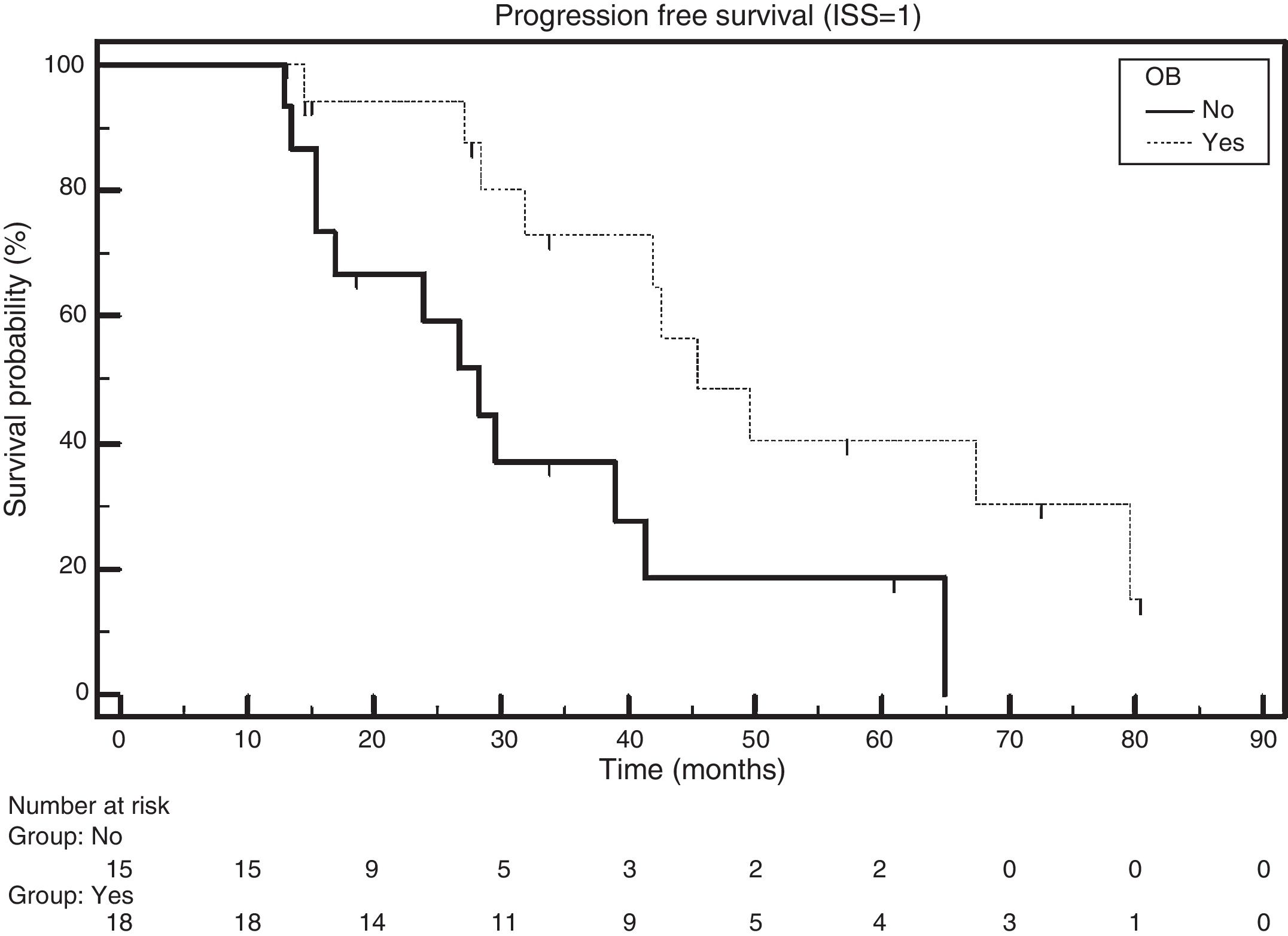
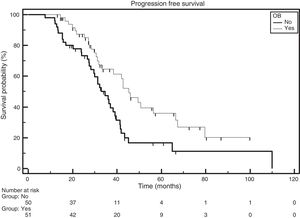
Patients with ISS 1 and the emergence of OB had a median PFS of 45.1 months, while those without the emergence of OB had a median PFS of 28.3 months (HR: 0.3720; 95% CI: 0.1505–0.9192; p-value=0.0120; Figure 2).
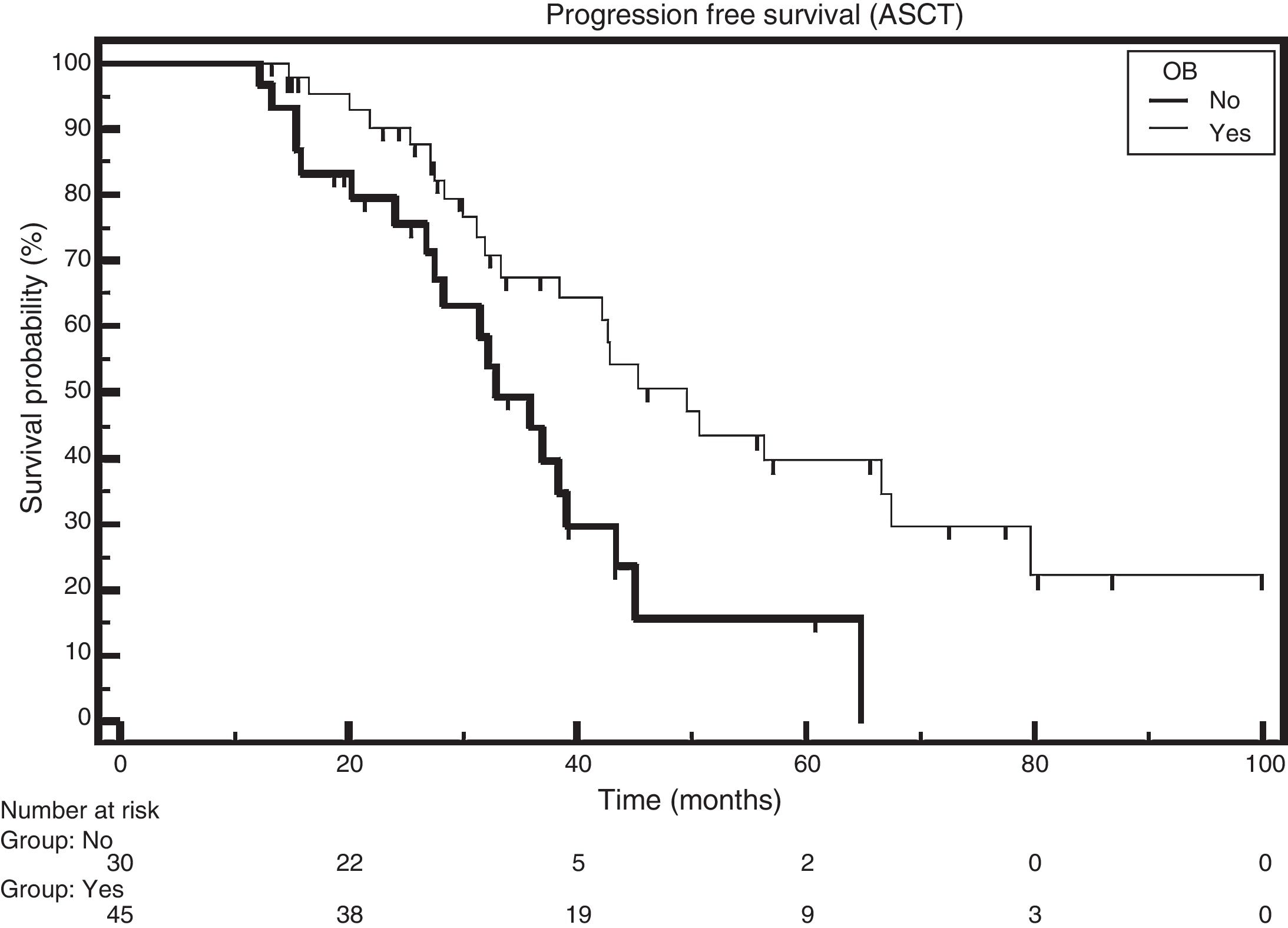
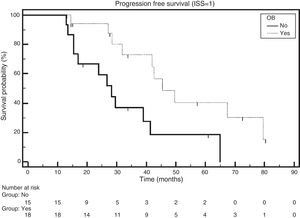
The median survival rate of patients who received ASCT and had the emergence of OB was 49.6 months, and for those without the emergence of OB the median survival rate was 32.8 months (HR: 0.4552; 95% CI: 0.2345–0.8835; p-value=0.0063; Figure 3).
The PFS was evaluated in multivariate analysis (Cox model) using ISS (1 vs. 2/3), response rate (CR vs. VGPR), type of treatment (transplant vs. no transplant) and emergence of OB (yes vs. no) as independent variables. In the analysis of the model that included (OB, Response Rate and ISS) only OB, and CR were independent predictors for better PFS. A second model was run using high-dose chemotherapy as a fourth variable, in addition to those described before. In this second model, the emergence of OB lost its prognostic significance, and the only variable significantly associated with increased PFS was CR.
DiscussionHere, we report the first Brazilian study involving the emergence of OB in MM patients after conventional chemotherapy or high-dose chemotherapy followed by ASCT. A prevalence of 50.5% of OB emerged in MM patients with VGPR or better, and the emergence of OB was more common in patients who underwent transplantation and in those who achieved CR. Previous studies reported a prevalence of the emergence of OB in transplanted patients, ranging from 5.9% to 24.5% and likewise, this study found that the rates of the emergence of OB were higher in transplanted patients.3,7,8,12 The use of novel agents in both transplant and non-transplant settings was also associated with a high prevalence of OB.4,5,13
The impact on overall survival could not be proven, possibly due to the relatively small sample size. Studies with larger sample sizes have shown longer overall survival among individuals presenting the emergence of OB.2,3,8,13 In one study involving 1942 patients, Wadhera et al.3 found significantly greater overall survival among those who developed secondary monoclonal gammopathy of undetermined significance (sMGUS) compared to those who did not (73 vs. 38 months, respectively).
In this study, PFS was superior in patients who developed OB, a finding that has been reported in previous studies assessing transplanted patients only2,9,10,13 and in studies involving patients who had undergone ASCT or not.7,12
It is well established in the literature that better response predicts superior survival.14–16 This study selected the emergence of OB among patients with better responses (at least VGPR) and even in this specific group of patients, the emergence of OB had a favorable impact on PFS. Analyzing PFS in a Cox regression model including ISS (1 vs. 2/3) and response rate (CR vs. VGPR) as well as the emergence of OB, the latter remained an independent variable to predict PFS. However, on adding the type of treatment performed (ASCT vs. no ASCT) to the model, only CR was significant even though the OB was close to the significance limit (p-value=0.07). The size of cohort could have influenced this result.
Fujisawa et al. failed to confirm improvement in survival assessing the impact of OB on subgroups with at least VGPR or CR in patients submitted to ASCT or otherwise, and similarly, no impact was found for the subgroup of patients eligible for ASCT. The authors questioned the potential prognostic factor of oligoclonality, specifically, that it might be more closely linked to better responses than to the emergence of OB per se.7
These conflicting results, both in terms of prevalence and impact on survival, are probably attributed to the heterogeneity of the studies published to date. Most studies had a retrospective design, different population sizes, different definitions of OB, frequency of SPE and immunofixation tests, and used different treatments.
The detection of these new monoclonal peaks in SPE or by immunofixation during the follow-up of MM patients can be mistaken for relapse and/or disease progression, leading to unwarranted treatment or changes in treatment. As such, the finding of oligoclonality, a small monoclonal component different from that identified at diagnosis, must be carefully interpreted and regular follow-ups should be carried out before treatment decisions are made.
The advances in the MM treatment have led to better responses. The incidence of the emergence of OB has increased and its impact on survival should be confirmed in large prospective studies.
ConclusionA high prevalence of the emergence of OB was seen in patients who had at least VGPR after conventional or high-dose therapy. This frequency was greater in patients who underwent ASCT and in those with CR. CR was more important than the emergence of OB on the PFS.
Conflicts of interestThe authors declare no conflicts of interest.