Despite knowledge advances on extramedullary haematopoiesis (EMH) in thalassemic patients, the real picture remains an open issue.
ObjectivesTo assess EMH prevalence in patients with thalassemia major (TM) and intermedia (TI), to describe magnetic resonance imaging (MRI) findings and to explore clinical risk factors.
MethodsIn this cross-sectional study, images and clinical records of 184 consecutive patients with thalassemia who underwent T2* MRI between 2004 and 2011 were reviewed. Association of EMH with survival was investigated for patients with available follow-up charts.
ResultsEMH was detected in 16/168 (9.5%) patients with TM (aged 19-49 years) and in 3/16 (18.8%) with TI (aged 36-41 years). Most (88%) had paravertebral thoracic and/or abdominal masses. Age was significantly associated with EMH risk (hazard ratio, [HR] 1.10/year; confidence interval [CI]: 1.03-1.18; p-value < 0.001), while lower pancreatic iron content by T2*MRI (HR: 0.94/ms; CI: 0.89-0.99; p-value = 0.049) was a protective factor. Estimated survival rate was superior for EMH-positive (n = 19) when compared to EMH-negative patients (n = 75) (p-value = 0.013).
ConclusionsThe prevalence of EMH was 10.3% (19/184), presented mainly as tumoral masses of 3 to 10 cm. Age was a risk factor for EMH development, while lower pancreatic iron might be a protective factor in this cohort.
Extramedullary haematopoiesis (EMH) is defined as the presence/growth of haematopoietic tissue outside the bone marrow.1-4 The pathogenesis of EMH has been attributed to the stimulation and differentiation of “latent haematopoietic niches” outside the bone marrow, usually located in the liver, spleen, lymph nodes, and aortic-gonad-mesonephros spaces, that remained quiescent after embryonic/foetal development.4-9 This condition could affect at risk patients, such as those diagnosed with thalassemia Major (TM) or thalassemia Intermedia (TI), in whom chronic anaemia, hypoxemia, ineffective erythropoiesis, and high erythropoietin may interact together with EMH development.5,6,8-10
Historically EMH was considered a rare event but nowadays it is increasingly being recognized, as more accurate diagnostic tools have become available and been incorporated in the clinical practice, including magnetic resonance imaging (MRI).4,11-14 Studies reported the prevalence of EMH ranging from 2.2% to 25.0%, a variability that is likely due to heterogeneous patient cohorts assessed in different settings. 1-4,7,9,15,16
A systematic review, that united data from two important retrospective studies and seventy case reports, analysing a total of 253 patients with EMH and thalassemia, recently described the clinical features of EMH.4 This review observed that the mean age and mean haemoglobin level at presentation were 35.3 years old and 8.2 g/dL, respectively.4 Furthermore, two remarkable studies (OPTIMAL CARE2 and E-SAAN1) revealed that transfusion dependence, age, and thalassemia severity might be associated with the incidence of EMH.1,2 However, uncertainty and heterogeneity remain regarding epidemiological aspects, risk factors, clinical presentation and the optimal treatment approach.1,2,4
Other factors associated with an increased prevalence of EMH have been identified: the IVS–I–6-mutated allele, splenectomy, non-transfusion dependent phenotype, skeletal deformities, lower myocardial iron content, and higher levels of the soluble form of transferrin receptor (sTfR) and of growth differentiation factor 15 (GDF-15).3,5,10,11,15,16
The clinical presentation of EMH usually manifests as thoracic or abdominal masses, from asymptomatic cases to severe organ impairment due to tumour compression of adjacent structures including organs such as the spinal cord, lung, heart, or large central vessels. 4,12,13,14,17 Signs and symptoms of liver and/or spleen impairment may be present due to haematopoietic tissue expansion.18,19 Recurrent haemorrhagic or exudative pleural effusions have also been described.20
Imaging tests such as MRI and computed tomography (CT) have demonstrated good accuracy to diagnose EMH. 4 However, in a few patients, other invasive tests such as biopsy may be necessary to confirm EMH and exclude differential diagnoses such as mesenchymal tumours, neuronal tumours, lymphomas, hematomas, other causes of serositis, liver diseases and splenomegaly.4,9,14,18
The optimal treatment approach is not clearly defined. Studies have reported combined therapies based on radiotherapy, hydroxyurea, and higher haemoglobin transfusion thresholds.2,4,9 Some cases may need surgery or urgent spinal cord decompression.4 Recently, Janus kinase (JAK) inhibitors have also been used in the treatment of EMH with encouraging results.21
The objective of the current study was to describe the EMH clinical, epidemiological and MRI findings in patients with TI or TM and to explore potential risk factors for its occurrence.
MethodsDesign, settings and ethicsThis study was a cross-sectional evaluation of consecutive patients with thalassemia major and intermedia from seven hematologic centres in Brazil referred for MRI iron load assessment at a reference centre (Hospital Israelita Albert Einstein, São Paulo), between 2004 and 2011. The participating centres were: Hospital Israelita Albert Einstein, São Paulo, SP; Fundação de Hematologia e Hemoterapia de Pernambuco - HEMOPE, Recife, PE; Centro de hematologia de São Paulo, SP; Hemocentro de Marília, Marília, SP; Centro Infantil Boldrini Campinas, SP; Centro de Hematologia e Hemoterapia do Paraná - HEMEPAR, Curitiba, PR and Universidade de Campinas - UNICAMP, Campinas, SP.
The study was approved by the Health Research Ethics Committees of the hospitals and written informed consent was obtained from participants before any study procedures were performed. The study was supported/funded by the Brazilian Ministry of Health.
ParticipantsAll consecutive patients with thalassemia, aged seven years or older followed at the participant treatment centres, who were referred for MRI T2* iron assessment of the heart, liver, and pancreas were eligible for this study. All patients were treated by their own physicians and referred to the Oncology and Haematology Centre, Hospital Israelita Albert Einstein, São Paulo, to assess MRI findings, laboratory results and clinical data. Evaluations included symptoms, medication records, chelation therapy and transfusion requirements both according to the patient assessment in the reference centre and the treating physicians’ reports.
Criteria for not performing the MRI were claustrophobia, pregnancy, presence of a pacemaker or intracranial aneurysm clipping, and history of non-compliance to iron chelation therapy.
MRI technique and measurementsThe MRI equipment used to assess tissue iron content in all patients was a 1.5T scanner (General Electric, Milwaukee, WI, USA). The MRI sequences were obtained using the multi-echo segmented fast gradient echo pulse (MFGRE) with the following parameters: FOV 44 cm, PFOV = 0.7, slice thickness 10 mm, spacing 8.0 mm, 4 slices, TR = 34 milliseconds (ms), FLIP 20 degrees, TE minimum of 2 ms, TE maximum of 16 ms, 8 echoes with 2 ms inter-echo, frequency matrix = 128, phase matrix = 128, NEX = 1, receiver bandwidth = 62.5 kHz; post-processing. T2* decay fitting was performed with Report Card V3.3 software (General Electric, Milwaukee, WI, USA) using the following formula: S = A*e (-TE/T2*) + C. The initial scaling factor was 25 ms, and a two-parameter fit was used (C = 0).
The following equation was used to calculate liver iron concentration (LIC) from measured T2*/R2*: [Fe]R2* = 0.0254 x R2* + 0.202. The same parameters were used to assess the myocardium (short-axis view focused on the cardiac interventricular septum). The myocardium sequence measures were analysed using the Report Card software.
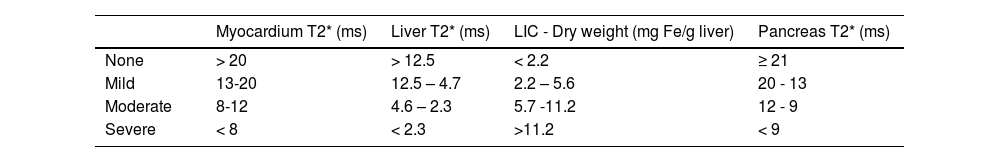
The MRI radiologic assessments and reports were performed by two experienced radiologists, who reached a consensus for all cases on the calculation of the MRI T2* and on the diagnosis of EMH. Images with artefacts that prevented the analyses were not recorded. The hepatic and pancreatic relaxation times (T2* in ms) were assessed at the transversal plane of the upper abdomen. The paraspinal musculature signal intensity was also assessed to calculate the signal intensity ratio (SIR) between these muscles and the pancreas.9,22,23 The criteria used for defining iron overload of each organ has previously been described (Table 1).9,22,23
Diagnosis of chronic liver disease was based on the following established radiologic criteria: hypertrophy of the caudate lobe and lateral segments of the left lobe (segments II & III) with concomitant atrophy of the posterior segments (VI & VII) of the right lobe.24,25 Furthermore, the most common radiologic signs observed in cirrhosis were considered: nodular liver surface; hypertrophy of the left and/or caudate lobes, and atrophy of the segment IV; expanded gallbladder fossa and reduction of the medial segment of left hepatic lobe.24,25 Assessment of hepatomegaly (> 18 cm) was based on the oblique craniocaudal length, which has been demonstrated to correlate with liver volume (r = 0.83).25
Clinical and laboratory assessmentsBlood samples were drawn during the patient visit to the MRI reference centre to evaluate ferritin serum levels using the same equipment for chemiluminescence throughout the entire duration of the study.
Variables and statistical analysisThe variables under evaluation in this study were the severity of thalassemia (TM or TI), gender, age, serum ferritin levels, LIC, T2* MRI values in ms for the pancreas and heart, spleen evaluation by MRI (splenomegaly or splenectomy), diagnosis of chronic liver disease (based on radiologic criteria) and hepatomegaly.
The continuous variables were analysed by means, medians, ranges, interquartile range, and standard deviations:
- •
Serum ferritin – ng/mL,
- •
LIC – mg Fe/g liver dry weight,
- •
Cardiac – T2* ms and
- •
Pancreatic iron overload – T2* ms.
The Shapiro-Wilk method was applied to test normal distributions. The Student t-test was used to compare parametric data, whereas the Mann-Whitney (paired) and Wilcoxon rank sum (unpaired) tests were used to compare non-parametric data.
The univariate analysis of categorical data was achieved using the chi-square and Fisher exact tests; variables with p-values < 0.1 were selected for multivariate analysis. A combined analysis of multiple variables potentially correlated with EMH was carried out by logistic regression, where p-values < 0.05 were considered significant.
Overall survival was estimated for patients with available follow-up data up to October 2017 using the Kaplan-Meier method which was then compared by the log-rank test.
The STATA version 11 and the Statistical Package for the Social Sciences (SPSS) software were used for statistical analysis.
ResultsIn the study period, the MRIs and charts of 184 patients with thalassemia were reviewed in the centre. Of these, 168 patients had TM (92.0%) and 16 TI (8.0%); 82 were male (44.6%) with the median age being 22 years old (range 7-69; mean of 24 years). There was no reliable information in the medical records about the type of thalassemia (alpha or beta).
All but one patient had iron overload in at least one organ, predominantly hepatic (99%). Most patients were on a regular transfusion regimen (TM: 96%; TI: 50%) and being treated with chelation therapy (TM: 98%; TI: 86%); about 4% (n = 7) of the total cohort did not have complete clinical data regarding their transfusion regimen and chelation therapy and therefore they were excluded from the analysis of these variables. The proportion of patients on each chelation medication was desferrioxamine (64/36.2%), deferasirox (48/27.1%), deferiprone (17/9.6%), and a combined therapy of desferrioxamine and deferiprone (48/27.1%).
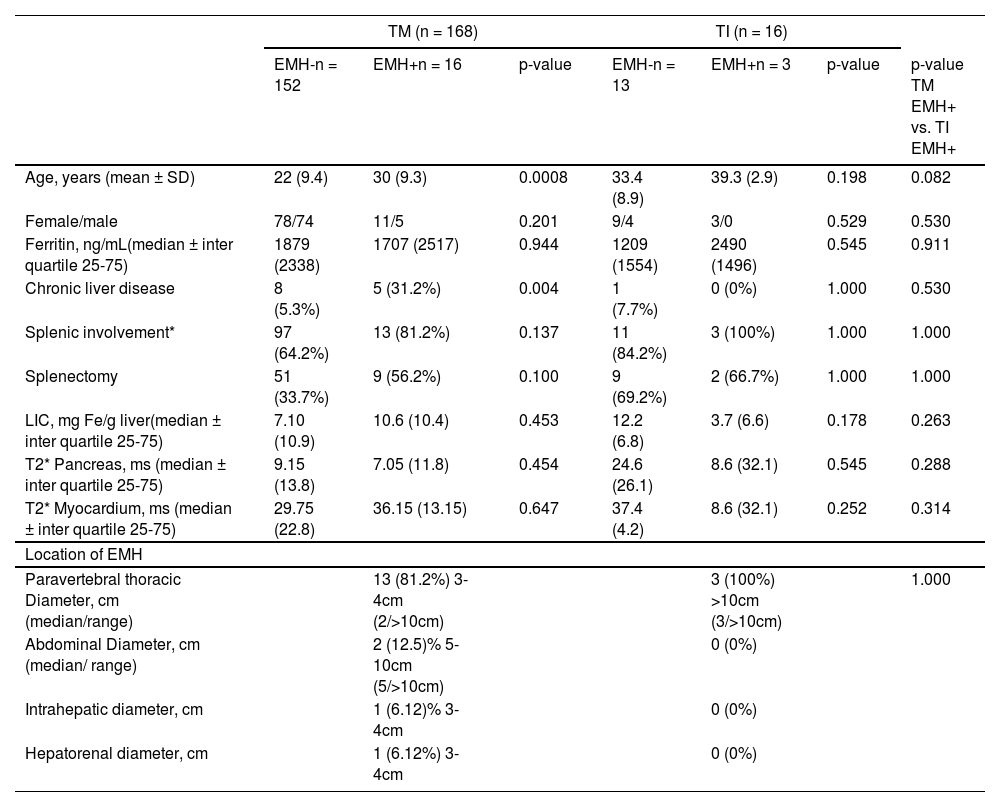
A total of 19 (10.3%) EMH cases were identified and distributed as 16 (9.5%) cases of TM patients and three (18.7%) cases of TI. The clinical characteristics of patients with or without EMH are provided in Table 2.
Demographics and clinical characteristics of patients with thalassemia major (TM) or intermedia (TI) with (EMH+) or without (EMH-) extramedullary haematopoiesis.
| TM (n = 168) | TI (n = 16) | ||||||
|---|---|---|---|---|---|---|---|
| EMH-n = 152 | EMH+n = 16 | p-value | EMH-n = 13 | EMH+n = 3 | p-value | p-value TM EMH+ vs. TI EMH+ | |
| Age, years (mean ± SD) | 22 (9.4) | 30 (9.3) | 0.0008 | 33.4 (8.9) | 39.3 (2.9) | 0.198 | 0.082 |
| Female/male | 78/74 | 11/5 | 0.201 | 9/4 | 3/0 | 0.529 | 0.530 |
| Ferritin, ng/mL(median ± inter quartile 25-75) | 1879 (2338) | 1707 (2517) | 0.944 | 1209 (1554) | 2490 (1496) | 0.545 | 0.911 |
| Chronic liver disease | 8 (5.3%) | 5 (31.2%) | 0.004 | 1 (7.7%) | 0 (0%) | 1.000 | 0.530 |
| Splenic involvement* | 97 (64.2%) | 13 (81.2%) | 0.137 | 11 (84.2%) | 3 (100%) | 1.000 | 1.000 |
| Splenectomy | 51 (33.7%) | 9 (56.2%) | 0.100 | 9 (69.2%) | 2 (66.7%) | 1.000 | 1.000 |
| LIC, mg Fe/g liver(median ± inter quartile 25-75) | 7.10 (10.9) | 10.6 (10.4) | 0.453 | 12.2 (6.8) | 3.7 (6.6) | 0.178 | 0.263 |
| T2* Pancreas, ms (median ± inter quartile 25-75) | 9.15 (13.8) | 7.05 (11.8) | 0.454 | 24.6 (26.1) | 8.6 (32.1) | 0.545 | 0.288 |
| T2* Myocardium, ms (median ± inter quartile 25-75) | 29.75 (22.8) | 36.15 (13.15) | 0.647 | 37.4 (4.2) | 8.6 (32.1) | 0.252 | 0.314 |
| Location of EMH | |||||||
| Paravertebral thoracic Diameter, cm (median/range) | 13 (81.2%) 3-4cm (2/>10cm) | 3 (100%) >10cm (3/>10cm) | 1.000 | ||||
| Abdominal Diameter, cm (median/ range) | 2 (12.5)% 5-10cm (5/>10cm) | 0 (0%) | |||||
| Intrahepatic diameter, cm | 1 (6.12)% 3-4cm | 0 (0%) | |||||
| Hepatorenal diameter, cm | 1 (6.12%) 3-4cm | 0 (0%) | |||||
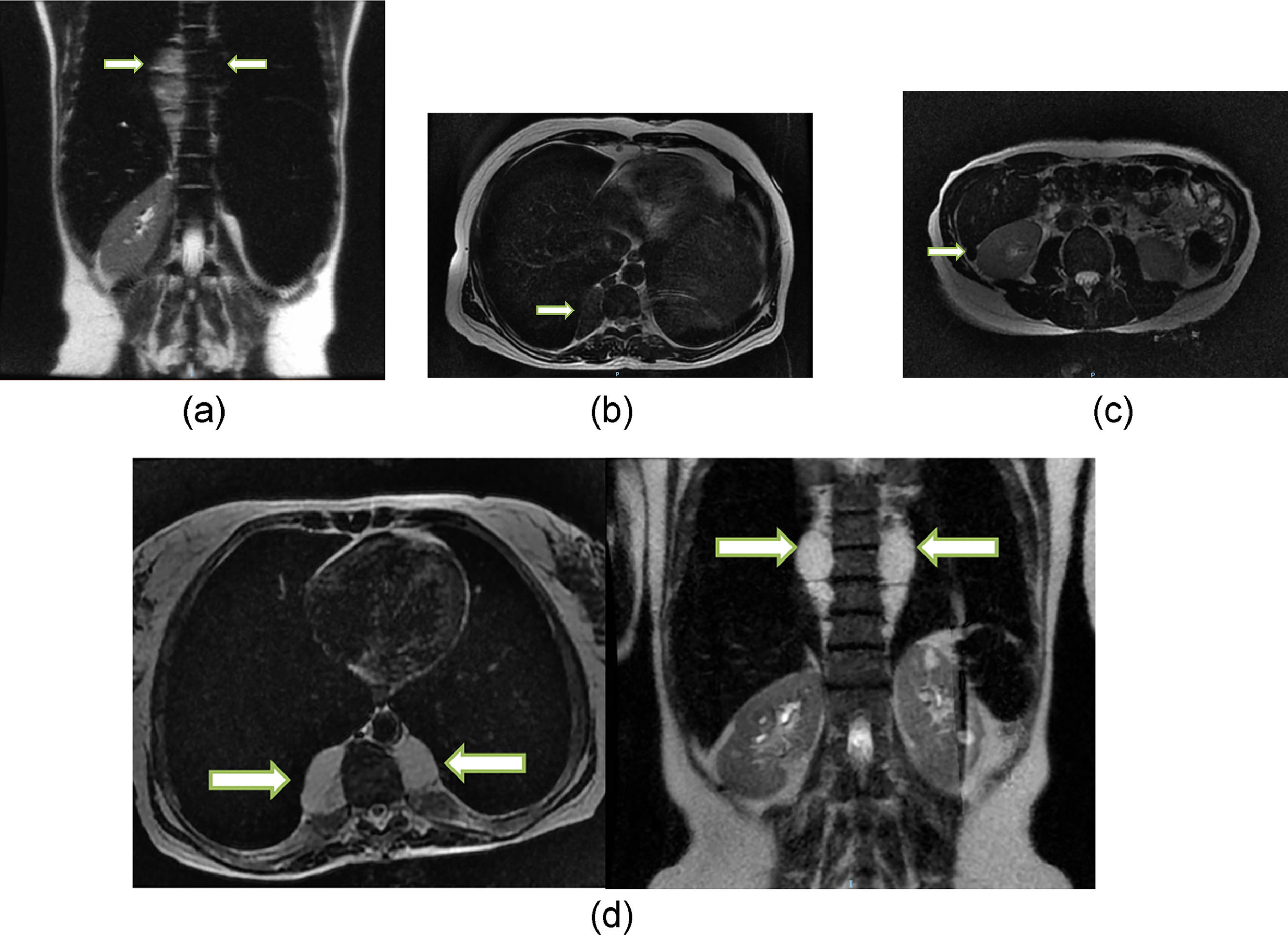
Among the EMH patients, 57.9% (n = 11) had masses that were < 5 cm in diameter, 21% (n = 4) between 5–10cm, and 21% (n = 4) >10 cm. Supplementary Table 1 details the features of the EMH patients. Four EMH images (Figures 1a-d) were selected to illustrate the radiologic characteristics of these patients. Figure 2 shows the anatomic distribution of EMH masses.
T2 fast spin echo magnetic resonance imaging (MRI) scans: A) Coronal scan of case 9, showing large soft tissue mass in the thoracic and lumbar paravertebral space (arrows); hepatosplenomegaly and marked hypo intensity of the liver and spleen, which indicates iron deposition. B) Axial scan of case 124 showing small soft tissue mass in the right thoracic paravertebral space (arrow). C) Axial scan of case 13 showing small soft tissue nodule in the hepatorenal space (arrow), presenting marked hypo intensity, indicating iron deposition. D) Axial and coronal of case 132 showing large soft tissue mass in the thoracic paravertebral space. Note the marked hypo intensity of the liver and spleen (coronal), which indicates iron deposition.
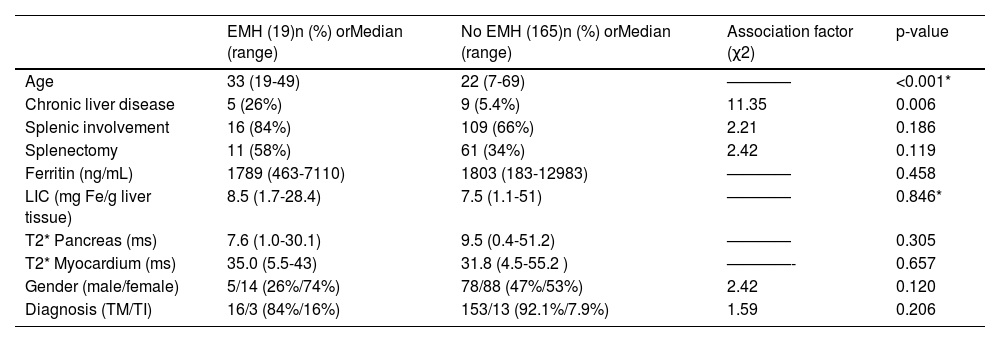
Univariate analysis showed a significant association between EMH and age (p-value < 0.001), with no cases of EMH in patients < 19 years old. A significant association was also observed between EMH and chronic liver disease (p-value = 0.006), as summarized in Table 3.
Potential Risk factors for EMH – univariate analysis.
EMH = extramedullary haematopoiesis; LIC = liver iron concentration; TM = Thalassemia Major; TI = Thalassemia Intermedia * Student t-test was applied to numeric variables with normal distributions confirmed by the Shapiro-Wilk test. Other numeric variables were analysed by the Wilcoxon-Mann-Whitney test and categorical variables by the chi-square or fisher exact test.
Multivariate analysis showed that EMH was significantly correlated with age (HR: 1.10/year; CI: 1.03-1.18; p-value < 0.001) as a risk factor, and with pancreatic T2* increment (HR: 0.94/ms; CI: 0.89-0.99; p-value < 0.049) as a protective factor. Twenty five percent (2/8) and 50% (2/4) of patients aged > 40 years with TM and TI, respectively had EMH. Only four patients with EMH did not have any pancreatic iron overload.
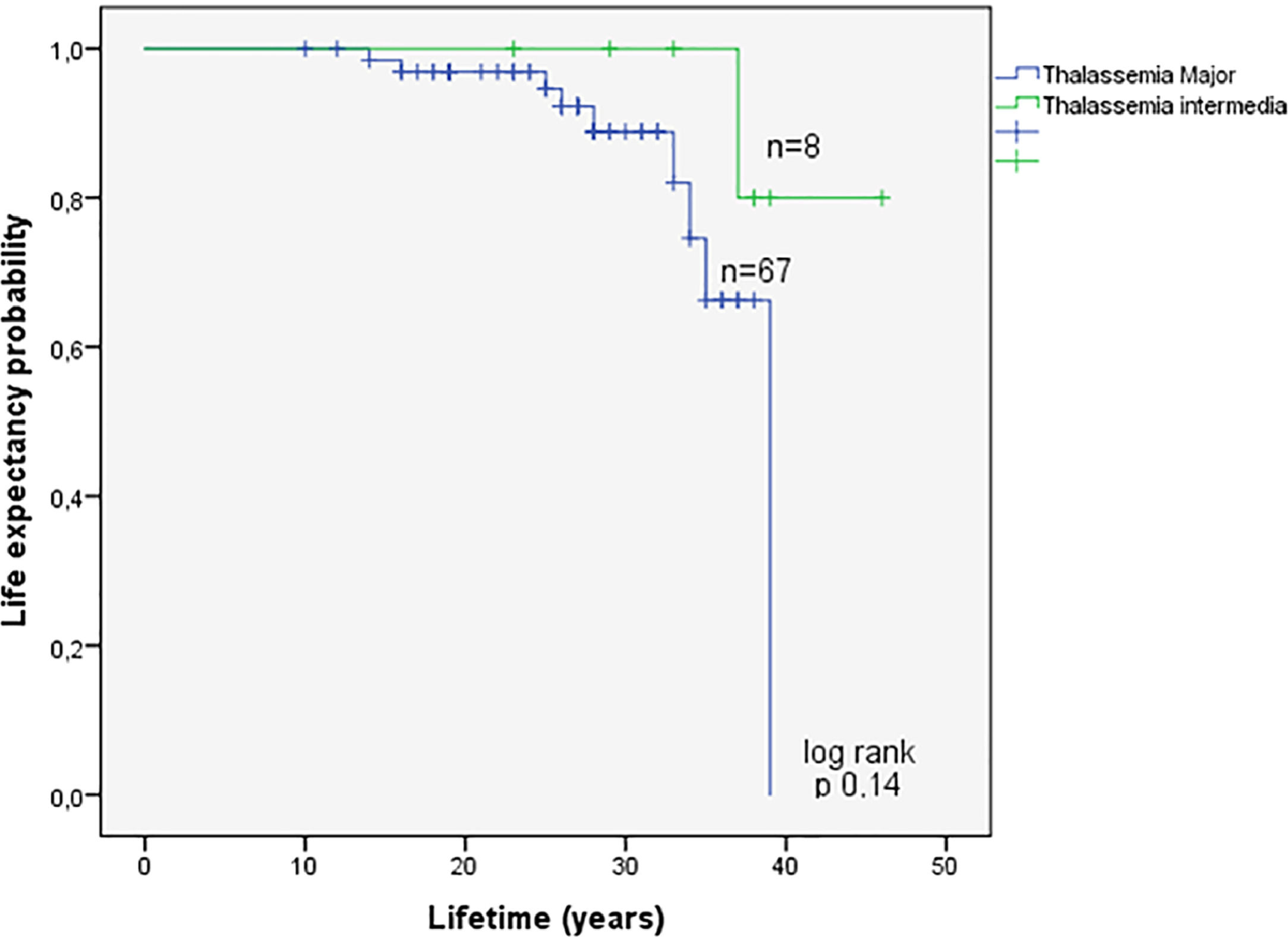
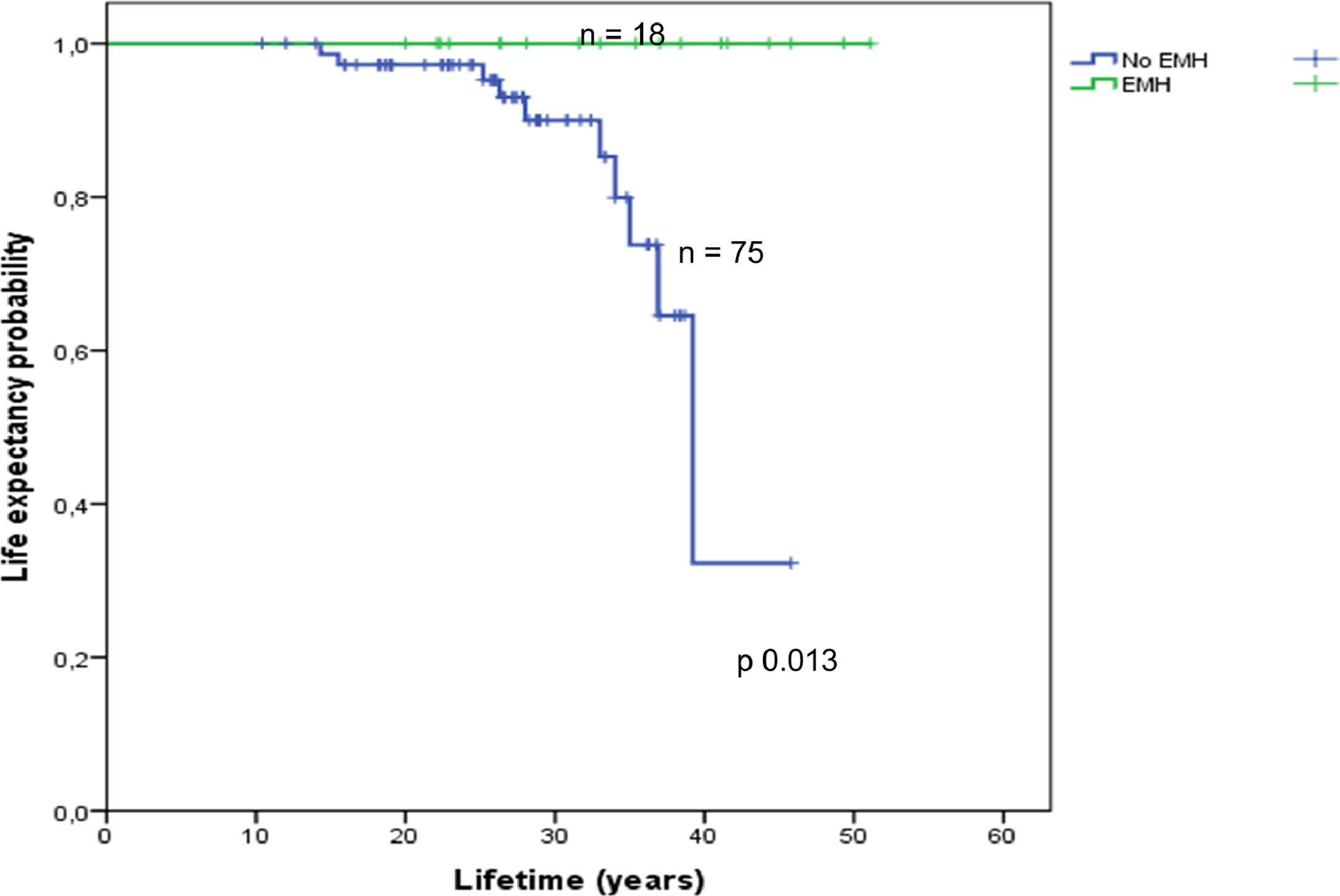
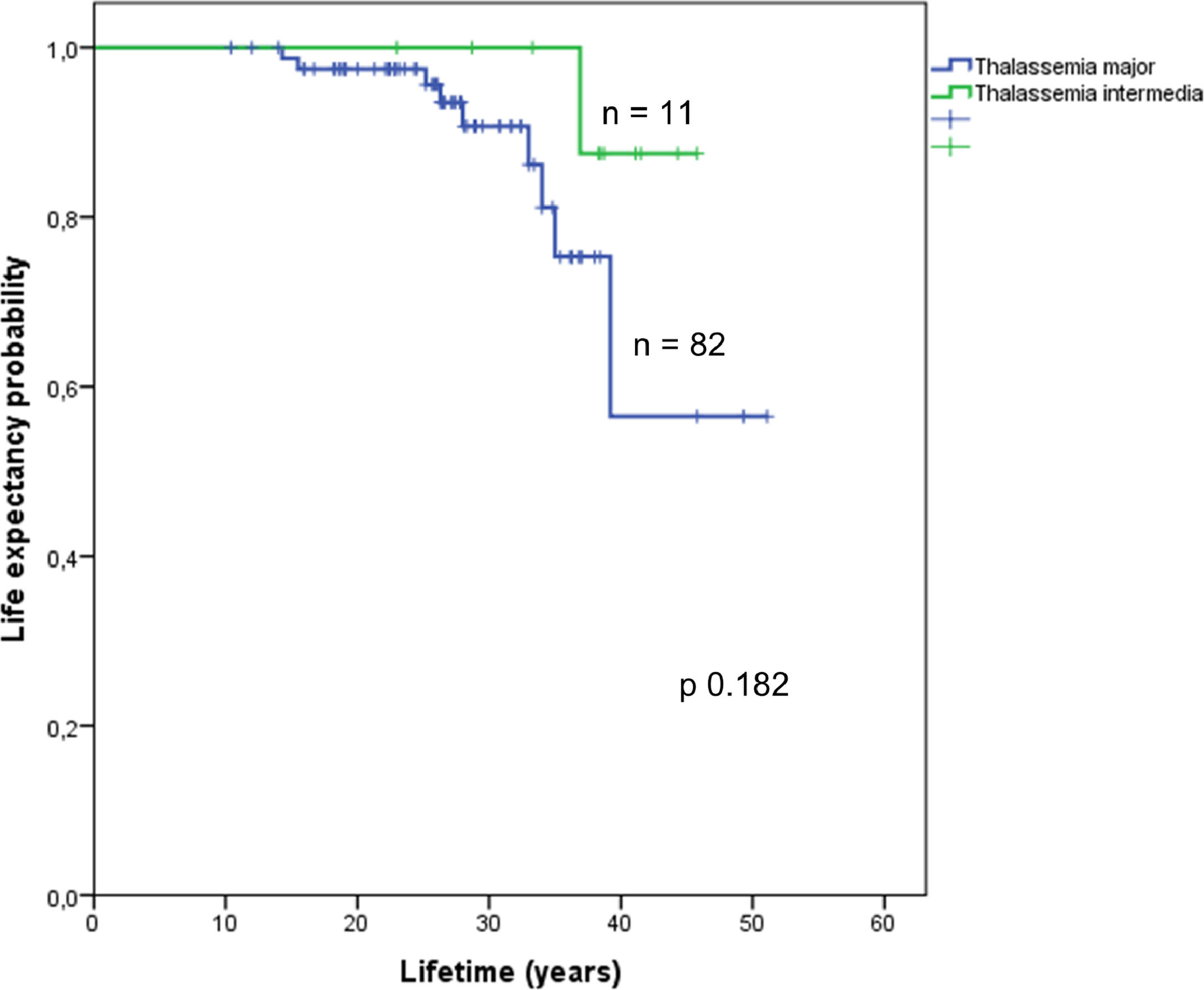
Assessment of survival in the EMH-free patients according to TM or TI is shown in Figure 3. Information on survival up to October 2017 was available for 94 (49%) of the studied population. The overall survival of 19 patients with EMH and the 75 patients without EMH was estimated using Kaplan-Meier curves (Figure 4). Overall survival was estimated for patients with available follow-up data up to October 2017 (19 EMH-positive patients and 75 in the EMH-negative group). There was a statistically significant difference in survival between patients with and without EMH (p-value = 0.013), possibly linked to existing baseline age differences at the study entry. Additionally, survival was compared between the TM (n = 82) and TI (n = 11) subgroups. Although the survival curves visually appear to be distinct (Figure 5), no statistically significant difference between them was found.
The prevalence of EMH observed in this cohort of patients with TM and TI was 10.3%, represented mostly by posterior thoracic masses of 5–10 cm. Age was strongly associated with higher risk to EMH, while chronic liver disease was only associated with EMH in the univariate analysis.
Literature on the clinical and epidemiological aspects of EMH has described massive marrow proliferation with extramedullary tumour masses in patients with homozygous beta-thalassemia inadequately transfused and even more frequently in patients with both TI (up to 20%) and hemoglobinopathies (E/beta).2,4,26 Another study observed a lower EMH prevalence (13.2%) in a large cohort of regularly transfused major thalassemic patients.15 In our cohort, the observed prevalence of EMH was 9.5% and 18.7% for TM and TI patients, respectively.
Besides transfusion dependence, age differences may also explain the disparities observed in EMH prevalence between studies; while a systematic review has shown a mean age of 35 years at EMH diagnosis, this study found median ages of 30 years and 39 years in the TM and TI subgroups, respectively.4,9 Moreover, one study of TI patients with low transfusion requirements reported an even higher EMH prevalence (21.2%), which increased up to 40% depending on the patients’ age.2 Other risk factors for EMH have been described in TI patients, such as low foetal haemoglobin and severe ineffective erythropoiesis.27 Splenectomy has been described as a protective factor for EMH development, but our study could not find such an association.2
There are few case reports of EMH in children and adolescents; one reports on low transfusion regimens in young patients.1,4,9 EMH was not observed in under 19-year-old patients in our cohort. EMH in older patients may be a consequence of a longer period of exposure to erythropoietic stimulation linked to the EPOR/JAK-STAT signalling pathway and differentiation of quiescent haematopoietic niches.1,2,4,6,9
Our study was not designed to explore correlations between transfusion regimen and EMH development due to the small percentage (50%; n = 8) of non-transfusion-dependent patients in the TI subgroup, however the literature addressing this issue suggests that non-transfusion-dependent TI are at higher risk of EMH. 1-8,11,13,15,26,27
Regular transfusion regimens may suppress erythropoietic stimulation thereby reducing the development of EMH as well as skeletal deformities in patients. 1-4,8,9,28 Moreover, there are observations of negative correlations between haemoglobin/erythropoietin ratios and markers of increased osteoclastic activity, such as the receptor activator of nuclear factor-kappa B ligand (RANKL), that suggest a similar etiopathogenic mechanism on bone remodelling may occur.28 However, correlations between EMH and skeletal changes in thalassemic patients is yet to be better explored in the literature.
In the current study, the main sites of EMH were thoracic and abdominal (88%), almost all of them in the thoracic paravertebral spaces as has previously been reported in thalassemic patients.1-4,26 Even so, clinicians should be aware that EMH may occur in less frequently reported sites, as was recently shown by a systematic review that revealed EMH cases in adrenal glands, the skull and even intracranial tissue.4
The survival data and the multivariate analysis must be interpreted cautiously, because EMH+ patients were already older than the EMH- patients in the enrolment phase of the study and there is uncertainty on the precise moment at which each patient developed EMH. Additionally, the EMH+ group may be more sensitive/responsive to erythroid factors, which could explain the better life expectancy observed compared to the EMH- group, however our data is limited and cannot conclude if EMH could really be positively influencing extended patient survival.
Our findings suggest that two factors might be linked to EMH: pancreatic iron T2* (ms) and chronic liver disease, while the latter was significant only in the univariate analysis. Higher pancreatic T2* MRI findings (lower pancreatic iron) was negatively associated with the presence of EMH in the multivariate analysis (HR: 0.94/ms; CI: 0.89-0.99; p-value = 0.049) which, although considered a statistically significant correlation, is a borderline result, that should be cautiously interpreted. This finding contrasts with two studies that observed lower/no cardiac iron overload in EMH-positive patients when compared to the EMH-negative group in a regularly transfused cohort, a subgroup which they named thalassemia intermedia-like pattern.6,15,16
The correlations between iron metabolism and EMH biology remain an open question. While excess exogenous iron appears to increase erythropoietic responsiveness and expansion of extramedullary erythroid mass in animal models of thalassemia29, iron overload may negatively affect effective erythropoiesis, but might be improved by iron chelation therapy by reducing reactive oxygen species (ROS).30
Our understanding is that patients with less risk of EMH and lower pancreatic iron concentration might have other protective factors that were not assessed in this study, such as higher mean haemoglobin levels, lower erythropoietin levels, and favourable pancreatic iron kinetics, similar to that observed in the myocardium in cases with the TI-like pattern.15,16
Regarding chronic liver disease as a risk factor for EMH, we consider that this could be a surrogate factor associated with increased age, higher LIC and hepatic fibrosis, but it could also be a consequence of microscopic foci of intrahepatic EMH inducing liver disease over the long term.18,19
The effective management of patients with thalassemia requires an optimal balance between iron chelation, haemoglobin target and organomegaly/EMH monitoring, organ dysfunction and bone health.4,9 Symptomatic EMH could be managed by surgery, irradiation, hydroxyurea and intensifying the transfusion regimen in TM patients.4,9
ConclusionThe prevalence of EMH was 10.3% in the current cohort, presenting mainly as tumoral masses (of 3 to 10 cm) located at the posterior mediastinum. Age was the main factor significantly associated with EMH risk. While lower pancreatic iron (higher T2* velocity - ms) might be a protective factor and chronic liver disease could be a risk factor for EMH, further studies are required. In this cohort, despite of analytic limitations, thalassemic patients who survived longer had a higher frequency of EMH. Further studies are needed to understand how EMH affects patient survival.
This study was funded by the Brazilian Ministry of Health. Also, this study acknowledge for the collaboration of ABRASTA (Brazilian Association of Thalassemia), ABRALE (Brazilian Association of Lymphoma and Leukemia) and its former president Merula Anargyrou Steagall.
Promotion statement: Ten percent or more of thalassemic patients may present extramedullary hematopoietic (EMH) lesions; they are usually located in thoracoabdominal paravertebral spaces with sizes 3-10 cm or more. Older age and chronic liver disease were associated with EMH risk, while lower pancreatic iron was protective. EMH is rare in patients younger than 19 years old. Non-transfusional-dependent beta-thalassemic patients might also have a higher prevalence of EMH, which may be associated with increased survival.