Although the efficacy of hydroxyurea (HU) in inhibiting erythrocyte sickling has been well demonstrated, the action of this drug on human neutrophils and the mechanism by which it improves the manifestations of the disease have not been studied thoroughly. We aimed to investigate the cell viability, along with inflammatory and oxidative markers in the neutrophils of sickle cell anemia (SCA) patients and the effects of HU therapy on these cells, by evaluating the dose-responsiveness.
MethodsIn the present study, 101 patients (45 men and 56 women, aged 18–69 years) with SCA were divided into groups according to the use or not of HU: the SS group (without HU treatment, n = 47) and the SSHU group (under HU treatment, n = 54). The SSHU group was further stratified into subgroups according to the daily dose of the drug that patients already used: SSHU - 0.5 g (n = 19); SSHU - 1 g (n = 26) and SSHU - 1.5–2 g (n = 9). A control group (AA) comprised 50 healthy individuals. Neutrophils isolated from whole blood were analyzed using Trypan Blue, monoiodotyrosine (MTT) and lactate dehydrogenase (LDH) toxicity assays. Myeloperoxidase (MPO), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activities and concentrations of interleukin 10 (IL-10), tumor necrosis factor alpha (TNF-α) and malonaldehyde (MDA) were also measured.
ResultsNeutrophils from SCA patients showed membrane fragility and a significant decrease in cell viability when analyzed by Trypan Blue (p < 0.05), MTT (p < 0.001) and LDH (p = 0.011), compared to the AA group. Levels of inflammatory (MPO, TNF-α, and IL-10) and oxidative markers (SOD, GSH-Px, and MDA) were also altered (p < 0.05) in these cells, showing a significant difference in the SSHU-1g and SSHU - 1.5–2 g groups, compared to the SS group. Treatment with HU reverted the levels of all markers to concentrations similar to those in healthy individuals in a positive dose-effect relationship.
ConclusionThe HU did not generate a cytotoxic effect on neutrophils in SCA patients, but it modulated their oxidative and inflammatory mechanisms, promoting cytoprotection with a positive dose-effect.
Sickle cell anemia (SCA) is the most prevalent hereditary hematological disease worldwide, resulting from a point mutation in the β-globin gene.1 SCA is primarily a disorder of erythrocytes, although studies have shown that neutrophils seem to play a prominent role in the pathophysiology of the disease and may be involved in both the initiation and propagation of vaso-occlusive crises (VOCs) and inflammatory responses.2–4
While the adhesion of erythrocytes to the vascular endothelium of postcapillary venules is known to be a crucial event in the pathophysiology of vaso-occlusion, animal models suggest that augmented leukocyte adhesion to the endothelium, especially neutrophils and the consequent formation of heterocellular aggregates may initiate this process. Importantly, the presence of neutrophils at the vaso-occlusive site, in addition to participating in the physical obstruction of the vessel, mainly due to the fact that they are relatively large cells (12−15 µm) and rigid, probably also makes a considerable contribution to local inflammation and reduction of the blood flow due to the production of inflammatory mediators and stimulation of adhesion molecules.2,5,6
Sickle neutrophils have a greater potential for response to inflammatory stimulus, which leads to increased respiratory burst and rapid adhesion to CD18 ligands, including intercellular adhesion molecule-1 (ICAM-1) on the endothelium and ICAM-4, higher avidity in adherence to vascular regions, thus propagating VOCs.2,7
These cells can also induce the formation of reactive oxygen species (ROS), oxidative stress, endothelial injury, reduction of nitric oxide (NO) bioavailability and vascular intimal hyperplasia.7,8 These changes are characterized by dysregulated production of cytokines and other mediators, such as tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), myeloperoxidase (MPO) and E and P-selectin. It can also cause an imbalance between the antioxidant system defenses, such as catalase, glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) and the produced ROS, with a predominance of oxidizing agents.8 These events, in turn, induce pancellular activation and the maintenance of a vicious cycle, promoting organ dysfunction or organ failure.8
Sickle neutrophils show dysfunctional apoptosis and abnormal survival time, which can alter cell function and increase the potential for damage.9 Such evidence suggests the role of neutrophils in modulating SCA. However, the mechanisms responsible for such complications have not been completely elucidated.5
Advances in disease management have radically changed the life expectancy of patients, from 14 (1973) to an average of 60 years (currently), particularly due to the available therapies.10 HU is considered the most promising among the new therapies. It effectively changes the course of the disease, improves quality of life and decreases mortality. It also increases fetal hemoglobin (HbF) concentration and erythrocyte hydration, reduces the expression of adhesion molecules and increases the synthesis and bioavailability of NO.11
Some studies have highlighted the decrease in neutrophil count in peripheral blood as the most prominent beneficial effect of HU, suggesting that neutrophils may be a preferred drug target. Although the efficacy of HU in inhibiting erythrocyte sickling has been well demonstrated, the action of this drug on neutrophils and how it improves disease manifestations have not been studied in depth.6,12
In our recent study, we demonstrated that HU has a protective action on sickle neutrophils.13 However, there is a risk of DNA damage associated with its exposure, with DNA Damage Index (DI) values significantly higher in HU-treated patients for longer periods (> 20 months). In this context, we investigated the neutrophilic response in SCA patients, with and without HU therapy, using markers related to toxicity, inflammation and oxidative stress.
MethodsStudy design and patient population selectionThis research is based on a cross-sectional, analytical, and observational study. It compared the behavior of neutrophils and mediators produced by them between SCA patients and healthy individuals and studied the effects of HU treatment on the disease.
Whole blood samples were collected from 101 adult patients (aged 18–69 years) of both genders (45 men and 56 women) who were diagnosed with SCA and were undergoing treatment at a reference university hospital in northeastern Brazil. All patients were in steady-state disease according to the Ballas criteria14 and were stratified into two groups: the SS group (without HU treatment) (n = 47) and the SSHU group (patients treated with HU) (n = 54). For the SSHU group, treatment with HU consisted of using a dosage of 15–30 mg/kg/day by administering one to four capsules of the drug (each capsule containing 500 mg or 0.5 g of HU), totaling 0.5 g–2 g daily, for at least 6 months. For some analyses, the SSHU group was further stratified into subgroups according to the daily dose of the drug that patients already used: SSHU - 0.5 g (n = 19); SSHU - 1 g (n = 26) and SSHU - 1.5−2 g (n = 9). A control group (AA) comprised 50 healthy individuals (blood donors). The exclusion criteria adopted for all groups were: the presence of infectious diseases, pregnancy, smoking, alcoholism and the use of an iron chelator or non-steroidal anti-inflammatory, immunosuppressive or antioxidants drugs. Samples of peripheral blood were collected in EDTA and heparin for the assays.
Isolation of polymorphonuclear leukocytesNeutrophils were obtained from whole blood through the gradient difference, using a 2.5% gelatin solution, in which erythrocytes were lysed with NH4Cl (pH 7.2), and the pellet, containing the polymorphonuclear cells, were resuspended in Hank's buffered saline solution.15 The suspension of isolated polymorphonuclear cells contained an average of 90% neutrophils, with the standard deviation of 2.9, estimated by optical microscopy.
Evaluation of neutrophil cytotoxicity in SCA patientsEvaluation of cell viability by Trypan BlueAfter the addition of 100 μL of a 0.1% Trypan Blue dye solution to the same volume of cell suspension (5.0 × 106 cells/mL), neutrophils were counted in a Neubauer chamber and assessed for their viability, which was estimated by counting 200 cells.16
Cell viability by MTT assayThe neutrophil suspension (5.0 × 106 cells/mL) was incubated for 30 min at 37 °C in the presence of Hank's buffered solution and then incubated with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) for 3 h in a 5% CO2 atmosphere. Cells were washed with PBS and 150 μL of DMSO was added to solubilize the formazan crystals. The absorbance was measured at 595 nm.17
Lactate dehydrogenase (LDH) activityThe neutrophil suspension (2.5 × 106 cells/mL) was incubated in the presence of Hank's buffered solution for 30 min at 37 °C, followed by centrifugation. The LDH activity was determined in the supernatant according to the manufacturer's instructions (Labtest Diagnóstica, Lagoa Santa, Minas Gerais, Brazil). The pyruvate to lactate conversion was monitored by measuring the consumption of NADH at 340 nm.
Study of inflammatory markers in neutrophils from SCA patientsMeasurement of cellular degranulationThe MPO concentrations were determined from the supernatant of a 5.0 × 106 cells/mL suspension according to the method described by De Young et al.18 Briefly, 100 μL of PBS, 50 μL of phosphate buffer, 20 μL of H2O2 (0.012%) and 20 μL of 3,3′,5,5′-tetramethylbenzidine (TMB-1.5 mM) were added to the suspension. The reaction was stopped after 3 min of incubation at 37 °C by the adding 30 μL of sodium acetate. The absorbance was determined at 620 nm.
TNF-α and IL-10 concentrationsThe concentrations of TNF-α and IL-10 were measured from the supernatant of a 5.0 × 106 cells/mL suspension using commercial ELISA kits for TNF-α and IL-10 (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. The reaction was measured at 450 nm.
Study of oxidative stress in neutrophils in SCA patientsMeasurement of superoxide dismutase (SOD) releaseThe SOD activity in the cell suspension (5.0 × 106 cells/mL) was determined using the RANSOD® Kit (RANDOX BRASIL Ltda., São Paulo, São Paulo, Brazil) according to the manufacturer's specifications. The SOD activity was measured by the degree of inhibition of this reaction at 505 nm.
Measurement of glutathione peroxidase activityThe GSH-Px was determined from the supernatant of a neutrophil suspension (5.0 × 106 cells/mL) according to the manufacturer's specifications for the RANSEL™-GLUTATIONA PEROXIDASE Kit (RANDOX BRASIL Ltda., São Paulo, SP, Brazil). The reaction was recorded at 340 nm by the decay of the absorbance in 2 min.
Malonaldehyde concentrationsThe MDA was determined from the cell supernatant (5.0 × 106/mL) by a stoichiometric reaction with thiobarbituric acid (TBA), after incubation at 100 °C/30 min to form a pink chromophore whose absorbance was measured in acidic solution at 560 nm.19
Statistical analysisThe GraphPad Prism 5.0 (USA) and SPSS (Statistic Package for Social Science, v.19.0) were used for the statistical analysis. The Kolmogorov–Smirnov test was used to verify the normality of the data and the mean values of the groups were compared using the analysis of variance (ANOVA), followed by Tukey, Kruskal–Wallis and Dunn's Multiple Comparisons tests, as required. Results are expressed as the mean ± standard error of the mean. Significance was established at p < 0.05.
Ethical considerationsThe study was approved by the Ethics Committee of the Federal University of Ceará (protocol number 101/12) and all patients signed a consent form.
ResultsTable 1 shows the clinical and laboratory characteristics of the study subjects, in which we observe an increase of 270% in the concentration of fetal hemoglobin (HbF) in patients treated with HU. We also demonstrate that the use of HU can promote an increase in the hemoglobin, hematocrit and mean corpuscular volume (MCV) levels and a reduction in the number of leukocytes and neutrophils.
Sociodemographic characteristics and clinical and laboratory data of patients with sickle cell anemia and healthy individuals (control group).
| AA (n = 50) | SS (n = 47) | SSHU (n = 54) | p-Value* | |
|---|---|---|---|---|
| Sex: Male/Female | 28/22 | 22/25 | 23/31 | – |
| Age (years) | 34.6 (18; 55) | 32 (18; 69) | 32.17 (19; 63) | – |
| Erythrocytes (×1012/L) | 4.5 (4.2; 5.2) | 2.66 (1.77; 4.0) | 2.61 (1.73; 3.82) | 0.724 |
| Hemoglobin (g/dL) | 13.5(12.2; 15.2) | 8.20 (5.57; 11.53) | 9.21 (5.5; 12.4) | 0.001 |
| Hematocrit (%) | 41 (37; 49) | 24.05 (16.9; 32.9) | 26.40 (17.6; 34.3) | 0.005 |
| MCV (fL) | 87 (79; 92.1) | 90.8 (74.4; 107.6) | 101.8 (79.7; 134.4) | <0.001 |
| MCH (pg) | 28.9 (25.9; 31.6) | 30.9 (24.4; 37.7) | 35.5 (27.3; 47.8) | <0.001 |
| Leukocytes (×109/L) | 5.1 (4.2; 8.4) | 12.29 (5.55; 22.10) | 9.83 (3.20; 19.70) | 0.002 |
| Neutrophils (%) | 47 (39; 51) | 59 (41; 85) | 49 (31; 72) | 0.048 |
| Reticulocytes (×103/µL) | NA | 272.5 (110.9; 597.8) | 235.4 (110.5; 433.3) | 0.518 |
| Platelets (×106/µL) | 220 (148; 312) | 371 (154; 798) | 388.2 (385; 880.4) | 0.904 |
| HbF (%) | NA | 5.6 (0.6; 13.8) | 15.12 (1.9; 38.7) | <0.001 |
AA: Control group (healthy individuals); SS: SCA patients not taking hydroxyurea; SSHU: SCA patients on hydroxyurea therapy; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; HbF: fetal hemoglobin; NA: not available. Data presented (except for M/F) as means (minimum; maximum). (*) p-value obtained using the Kruskal–Wallis test.
Table 2 shows that the number of viable neutrophils of the SS group was significantly lower than that of the AA group (p < 0.05). The number of viable neutrophils in the SSHU group was similar to the SS and control (AA) groups, i.e., there was no significant difference.
Evaluation of the influence of hydroxyurea therapy on the viability of neutrophils in patients with sickle cell anemia by Trypan Blue assay.
| Neutrophils (%) | ||
|---|---|---|
| Viable not viable | ||
| AA (n = 50) | 95,3 ± 0,33 | 4,7 ± 0,33 |
| SS (n = 47) | 93,7 ± 0,34a | 6,2 ± 0,35 |
| SHU (n = 54) | 95,0 ± 0,42 | 5,0 ± 0,42 |
AA: Control group (healthy individuals); SS: Group of patients with sickle cell anemia (SCA) not treated with hydroxyurea (HU); SSHU: Group of SCA patients treated with HU. Tukey’s test.
Table 3 shows the influence of HU therapy on cytotoxicity parameters in neutrophils in SCA patients. Cell viability analyzed by the MTT assay showed a significant difference (p < 0.001) between the SS and AA groups. A significant increase in viable neutrophils was observed in the SSHU - 1g and SSHU - 1.5–2 g groups, when compared to the SS group. There was also a significant increase in viable cells in the SSHU - 1g group, compared to the SSHU - 0.5g group.
Evaluation of cell viability of neutrophils in patients with sickle cell anemia treated or not with hydroxyurea by MTT and LDH tests.
| Parameters | AA (n = 50) | SS (n = 47) | SSHU (n = 54) | p-Value | ||
|---|---|---|---|---|---|---|
| SSHU - 0.5 g (n = 19) | SSHU - 1.0 g (n = 26) | SSHU - 1.5−2.0 g (n = 9) | ||||
| MTT (% of viable cells) | 57.46 ± 3.29 | 35.14 ± 2.32a | 35.48 ± 3.38 | 56.62 ± 6.35b,c | 50.98 ± 3.32b | <0.001 |
| LDH (U/L) | 6.62 ± 0.72 | 9.33 ± 0.81a | 6.93 ± 0.54 | 5.66 ± 0.61b | 4.27 ± 0.59b | <0.001 |
AA: Control group (healthy individuals); SS: Group of patients with sickle cell anemia (SCA) not treated with hydroxyurea (HU);
SSHU: Group of SCA patients treated with HU (in doses of 0.5 g, 1.0 g or 1.5−2.0 g/day). Analyses performed in triplicate for each sample. Results were expressed as mean ± SEM. ANOVA followed by Tukey’s test.
The LDH enzyme activity was statistically higher in the SS group, when compared to the AA group (p = 0.011) (Table 3). The SSHU - 1 g and SSHU - 1.5–2 g groups showed a significant reduction in LDH levels, compared to the SS group (p < 0.001).
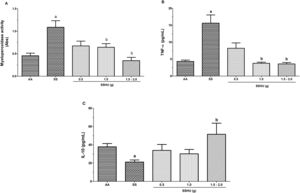
Evaluation of inflammatory markers in neutrophils in SCA patients treated or not with HUMean absorbance values of 0.45 ± 0.05 and 1.08 ± 0.14 for the AA and SS groups, respectively, demonstrated a significant increase in neutrophil degranulation and MPO concentration in the SS group (p = 0.001). The SSHU patients showed a mean of 0.67 ± 0.1; 0.64 ± 0.08 and 0.34 ± 0.07 for the SSHU - 0.5g, SSHU - 1g and SSHU - 1.5−2g groups, respectively. A significant reduction in the MPO concentration was observed in the SSHU - 1g and SSHU - 1.5−2g groups, compared to the SS group (p < 0.001) (Fig. 1A).
Evaluation of inflammatory markers in neutrophils of patients with sickle cell anemia (SCA) treated or not with hydroxyurea (HU). AA: Control group (healthy individuals); SS: Group of SCA patients not treated with HU; SSHU: Group of SCA patients treated with HU (in doses of 0.5 g, 1.0 g or 1.5–2.0 g/day). (A) MPO activity. (B) TNF-α concentration. (C) IL-10 concentration. Analyses performed in triplicate for each sample. (p-value-Kruskal–Wallis and Dunn’s Multiple Comparison).
ap < 0.05 vs AA group.
bp < 0.05 vs SS group.
Neutrophils in patients in the SS group showed a significant increase in TNF-α levels (15.62 ± 2.4 pg/mL), when compared to the AA group (4.31 ± 0.4 pg/mL) (p < 0.005). However, a significant reduction in TNF-α levels was observed in the SSHU - 1 g (3.77 ± 0.36 pg/mL) and SSHU - 1.5−2 g groups (3.59 ± 0.39 pg/mL), compared to the SS group. The TNF-α level in the SSHU - 0.5 g group was 8.21 ± 1.5 pg/mL (Fig. 1B).
Fig. 1C shows a significant reduction in IL-10 concentrations in the SS group (21.21 ± 2.2 pg/mL), when compared to the AA group (37.85 ± 3.5 pg/mL) (p < 0.005). Only neutrophils in patients in the SSHU - 1.5–2 g group (51.49 ± 12.1 pg/mL) demonstrated a significant increase in IL-10, compared to the SS group. The mean values of the SSHU - 0.5g and SSHU - 1g groups were 33.89 ± 6.5 and 30.19 ± 4.7 pg/mL, respectively.
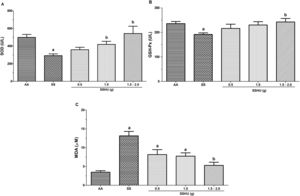
Evaluation of oxidative stress markers in neutrophils in SCA patients treated or not with HUThe measurement of the SOD enzyme release in the SS group (291 ± 20 U/L) was significantly lower, when compared to the AA group (498.7 ± 33 U/L). There was also a significant increase in the SSHU - 1 g (418.3 ± 35.1 U/L) and SSHU - 1.5−2 g (542.6 ± 82.1 U/L) groups, when compared to the SS group (p < 0.001). There was no significant difference between the SSHU - 0.5g (358 ± 27.5 U/L) and the other groups (Fig. 2A).
Evaluation of oxidative stress markers in neutrophils of patients with sickle cell anemia (SCA) treated or not with hydroxyurea (HU). AA: Control group (healthy individuals); SS: Group of SCA patients not treated with HU; SSHU: Group of SCA patients treated with HU (in doses of 0.5 g, 1.0 g or 1.5–2.0 g/day). (A) SOD activity. (B) GSH-Px activity. (C) MDA concentration. Analyses performed in triplicate for each sample. (p-value-ANOVA and Tukey's test).
ap < 0.05 vs AA group.
bp < 0.05 vs SS group.
A significant reduction in the GSH-Px activity was observed in the SS group (191.4 ± 6.4 U/L), compared to the AA group (235.7 ± 8.8 U/L) (p = 0.001). The SSHU - 1.5–2 g group (242.5 ± 14.2 U/L) demonstrated a significant increase in the GSH-Px, when compared to the SS group. The groups SSHU - 0.5g (216 ± 16.9 U/L) and SSHU - 1g (229.7 ± 12.5 U/L) did not show statistical differences in comparison to other groups (Fig. 2B).
The mean values of lipid peroxidation products, estimated by the MDA assay, showed a significant increase in the SS (13.15 ± 1.17 μM), SSHU - 0.5 g (8.15 ± 1.32 μM) and SSHU - 1 g (7.73 ± 0.88 μM) groups, when compared to the AA group (3.46 ± 0.40 μM) and a statistically significant reduction in the SSHU - 1.5−2 g group (5.27 ± 0.84 μM) in comparison to the SS group (p < 0.001) (Fig. 2C).
DiscussionAlthough the polymerization of HbS in erythrocytes is considered the main trigger for SCA, a few studies have investigated the role of neutrophils in the pathophysiological mechanism of the disease, as well as the influence of the HU treatment on cell function.
The vaso-occlusive events featuring SCA are multicellular phenomena with direct involvement of neutrophils, both in the beginning and in their propagation. Inflammatory and infection mechanisms induced by neutrophils participate in the vaso-occlusive processes. Furthermore, neutrophils in SCA patients exhibit greater adhesion and ability to interact with other cells, such as platelets, erythrocytes, and endothelial cells, which can promote thromboinflammatory events and damage to target organs.9
In this study, we observed that SCA patients had a significantly high leukocyte and neutrophil count, and that HU therapy not only increased the concentration of HbF, but also reduced the numbers of these cells, corroborating previous studies on the benefits of using HU.10,11,20 This change in the number of neutrophils can induce a decrease in disease severity, since neutrophilia is often associated with an increased risk of painful crises, stroke, acute chest syndrome and mortality.21 The HU can reduce granulopoiesis in the bone marrow and induce changes in the cell migration and neutrophilic apoptosis rate, thereby decreasing localized hypoxia and endothelial damage.11,20
To test the hypothesis that HU causes damage to the neutrophilic structure in SCA patients, we used the Trypan Blue dye as an indicator of membrane damage and the MTT assay to evaluate cellular metabolic function. In both cases, the neutrophil viability was reduced in SS patients, suggesting that the pathophysiological mechanisms of the disease itself alter cellular metabolism and induce damage to the plasma membrane. Cytotoxicity was not observed in HU therapy patients. However, a positive dose-effect association was observed, suggesting that HU preserves cell viability in neutrophils. Analyzing the toxicity of antiretroviral drugs, Foli et al. observed that HU alone does not alter cellular mitochondrial function, despite its cytostatic action.22 However, the combination of HU and some antiretrovirals can promote an increase in mitochondrial cytotoxicity due to the synergism of the drugs in use.22
Corroborating previous results, we demonstrated that HU can induce a cytoprotective effect on neutrophils in patients. It is inversely proportional with the dose of HU administered and the released LDH concentration by neutrophils. Plasma LDH levels are significantly higher in SCA patients.20,23 Its detection in extracellular fluid is indicative of cell death or loss of membrane integrity and is associated with an increased incidence of priapism, pulmonary hypertension and leg ulcers.20,23
HU has high biological potential and antineoplastic properties. However, its long-term effectiveness and safety are controversial. Some studies attribute genotoxic and teratogenic actions, while others report in vivo mutagenicity similar to that found in healthy individuals.24 Some aspects of HU therapy, such as optimal dosage and safe exposure time remain unclear. Evidence suggests that the effect on DNA methylation may depend on the drug concentration, exposure time and cell line, or even the individual response of each patient.25 In this study, we did not investigate the long-term effects.
Our analyses showed high levels of MPO and TNF-α in neutrophils and decreased levels of IL-10 in patients. These results demonstrate the important role of neutrophils in several events that promote the chronic inflammatory state of SCA. HU therapy had the capacity to revert the levels of these markers to values similar to those measured in healthy individuals.
Previous studies have demonstrated the role of MPO in the defense of the organism by reacting with H2O2 molecules to form hypochlorous acid, a potent oxidizing agent with antimicrobial activity.3,5,7 However, in situations of intense cellular activation, such as in SCA, MPO overactivity occurs, which in turn can activate the nuclear factor κB (NF-Kb) and TNF-α, induce lipid peroxidation and increase membrane permeability. These actions can contribute to the initiation and maintenance of chronic inflammatory processes and oxidative damage in SCA.3,5,7,21 HU, in addition to reducing the neutrophil count, can also reduce the neutrophil activation and release of pro-inflammatory and oxidative mediators.
Elevated concentrations of TNF-α have been implicated in several inflammatory diseases.21 This molecule participates in the upregulation of cytokine cascades, which can promote an increase in erythrocyte-leukocyte-endothelium interactions and changes in neutrophil apoptosis, resulting in an uncontrolled release of its cell contents.5,21
Lanaro et al. also demonstrated a significant increase in IL-10 in HU-treated patients and a positive correlation between the HbF concentration and IL-10.21 They also observed an overexpression of mRNA-encoding genes for IL-10 in neutrophils. These findings suggest that neutrophils are activated even at the steady state of the disease and that the use of HU can reduce degranulation, even when there is no decrease in the neutrophil count. HU can promote a qualitative change in the cell activity of sickle neutrophils, whose action would attenuate VOCs and various clinical signs.3,5,7
In this study, a significant reduction in the levels of the enzymes SOD and GSH-Px was demonstrated in the neutrophils of the SS group in relation to healthy individuals. The MDA concentrations in the neutrophils of these patients was increased by 360%. These results indicate a greater susceptibility of patients to vascular and organ damage due to the deficiency in the production/release of antioxidants or the fact that the enzymes may be being inhibited by the high oxidative burden generated by the disease. However, we also observed that HU reduced the oxidative stress generated by activated sickle neutrophils by the restoration of the measurement markers to values similar to those found in healthy individuals.
To date, very few studies have evaluated the activity of antioxidant enzymes in SCA (measured mostly in plasma and not in isolated neutrophils) and conflicting results have been reported.7,26 Cho et al. demonstrated a negative correlation between the levels of TNF-α and SOD and the levels of GSH-Px and LDH.27 The authors also reported that HU is capable of interfering in processes related to inflammation and oxidative stress in several cell lines, and not only in erythrocytes.27 The increase in HbF concentrations, the reduction of cell lysis and the effects on sickle neutrophils may be the main beneficial effects of HU.5,6,12
Notably, no significant difference was observed between patients in the SSHU - 0.5 g and SS groups. However, when analyzing the data from the MTT, LDH, MPO, TNF and SOD tests, we observed a significant difference in the neutrophil parameters of the patients in the SSHU - 1 g and SSHU - 1.5–2 g groups, compared to the SS group, expressing similar mean values to those found in healthy individuals (group AA). In the MTT test, a significant difference was also observed between the SSHU - 0.5 g and SSHU - 1 g neutrophils. In the analyses of IL-10, GSH-Px and MDA, only the SSHU - 1.5−2 g group showed significance, when comparing the groups under treatment with HU with the group without treatment.
HU therapy for SCA thus represents a challenging clinical paradigm whose action of the drug appears to be involved in several cell lines, including neutrophils. Because the beneficial effects of HU on laboratory parameters are dose-dependent and “more is better” when considering the therapeutic benefits of HbF induction, the optimal HU dose is what achieves maximal HbF without severe hematological or other toxicities.28,29 However, the lack of standardization regarding the optimal dosing, monitoring and dose escalation processes is also evident, especially regarding treatment targets and thresholds for toxicity29, including in the modulation of neutrophilic mechanisms.
Given historical concerns about the myelosuppressive effects of HU, older dosing strategies were conservative and almost always based on the patient weight, typically starting at 15–20 mg/kg/day, with subsequent trial-and-error-based dose escalation to obtain the maximum tolerated dose (MTD).29 Studies using HU at MTD typically achieve higher percentage HbF values than lower doses.28 In addition, these studies have argued that an individualized dosage of HU based on pharmacokinetics represents a more efficient strategy than traditional weight-based dosing and stepwise escalation to MTD.28,29,30 In the present study, our results demonstrated safety and favorable effects on the neutrophils of SCA patients using HU at higher doses.
ConclusionIn conclusion, our results demonstrate that the HU treatment does not exert a cytotoxic effect on neutrophils of SCA patients by the analyses investigated here. HU has been shown to be capable of modulating oxidative and inflammatory mechanisms related to neutrophils, in a positive dose-effect relationship, and may even promote a protective effect on these cells. However, despite evidence of the involvement of neutrophils in the SCA pathophysiology and the action of HU, additional investigations are needed to better understand the mechanisms by which HU acts on these cells and the question of the safety of its use, in relation to optimal dosage, long-term effects and the patient age, among other aspects.
Conflicts of interestThe authors declare no competing interests.
Sources of fundingThis research did not receive any specific grants from funding agencies in the public, commercial or not-for-profit sectors.
The authors would like to thank all patients and healthy volunteers who participated in this project. We are also grateful for the support of the National Council for Scientific and Technological Development (CNPq) and the Cearense Foundation of Support for Scientific and Technological Development (FUNCAP).