Low levels of neutrophils can be an intrinsic condition, with no clinical consequences or immunity impairment. This condition is the benign constitutional neutropenia (BCN), defined as an absolute neutrophils count (ANC) ≤2000 cells/mm. Diagnosis of BCN is of exclusion where patients are submitted to blood tests and possibly to invasive diagnostic search until secondary causes of neutropenia are ruled out. The natural history of the disease suggests benign evolution and Brazilian study showed an overall frequency of 2.59%. The main mechanisms include reduced neutrophil production, increased marginalization, extravasation to the tissues and immune destruction. Genetic studies showed strong association between the single nucleotide variant rs2814778 located on chromosome 1q23.2 in the promoter region of the atypical chemokine receptor 1 (Duffy blood group system) gene (ACKR1, also termed DARC) and BCN. The aim of this study is to evaluate FY phenotypes and genotypes including the analysis of the rs2814778 SNP in Brazilian patients with BCN in order to determine an effective diagnostic tool, allowing reassurance of the patient and cost reduction in their care.
MethodsCase control study, with 94 individuals (18 patients and 76 controls). Phenotyping was performed by gel test and genotyping was performed by PCR-RFLP.
ResultsWhite blood cell (WBC) and absolute neutrophils (AN) counts showed lower levels in patients compared to controls. In the patient group 83.3% were genotyped as FY*B/FY*B. The SNP rs2814778 (-67T > C) was identified in 77.8% of the patients genotyped as FY*B-67C/FY*B-67C. In the control group, 72.7% were homozygous for the wild type and 23.3% were heterozygous.
ConclusionThis study reinforces that FY phenotyping and genotyping can be used to detect most people with BCN, avoiding excessive diagnostic investigation. Besides, this procedure may reduce health costs and be reproductible in clinical practice.
White blood cell (WBC) count is a laboratorial tool ordered to assess immunocompetence, inflammation and infection. Within a normal range it is associated with a better immune response, however, low levels of WBC alert the clinician, beginning an invasive and costly diagnostic search.
Low levels of neutrophils can be an intrinsic condition, with no clinical consequences or immunity impairment and this condition is the benign constitutional neutropenia (BCN),the most common form of neutropenia, defined as an absolute neutrophils count (ANC) ≤2 103/μL,1,2 in more than one measurement, without recurrent infections or any other cause of neutropenia for at least one year of follow-up. Before the diagnosis, other causes should be excluded: chemotherapy, leukemia and other hematologic diseases, radiation therapy, splenomegaly, vitamin deficiencies, rheumatologic and infectious diseases.3
The natural history of the disease suggests benign evolution and the prevalence of common infections and diseases has shown to be low and does not differ from the general population (Lakhotia et al.4). However, the pathophysiology has been the subject of debates between authors. For Papadaki et al.5 and Addas-Carvalho et al.,6 the main mechanisms of neutropenia are the reduced neutrophil production by the bone marrow, the increased marginalization, the extravasation to the tissues and the immune destruction of mature cells. For Reich et al.,7 the low count does not result from adhesion to the endothelium of post-capillary venules, reduced cellularity or abnormal maturation and for Bashira et al.,8 inflammatory cytokines play a minor role.
Genetic studies showed strong association between the single nucleotide variant rs2814778 located on chromosome 1q23.2 in the promoter region of the atypical chemokine receptor 1 (Duffy blood group system) gene (ACKR1, also termed DARC) and BCN.8
The anthitetical Fya and Fyb antigens are encoded by two allelic forms of the FY gene, FY*A and FY*B. They differ by a single nucleotide variation at nucleotide 125 (c.125G > A), which changes p.Asp43Gly. Fy(a − b−) phenotype in Africans is due to a substitution from T to C at the GATA box motif of the FY*B promoter (c.-67T > C), which is the single nucleotide variant, rs2814778, of interest in this paper. This change disrupts the binding site for the GATA-1 erythroid transcription factor, resulting in a silent FY*B in the erythrocytes but not in other tissues. The substitution c.265C > T weaken the Fyb antigen and is associated with the phenotype known as Fyx.7,9,10
Bashira et al.8 associated the Fy(a − b−) phenotype with low WBC by the relative neutropenia hypothesis that erythroid ACKR1 null progenitors preferentially differentiate in myeloid cells. In this situation, the activated ACKR1 null neutrophils are retained in the spleen, resulting in relative neutropenia. For Reich et al.,7ACKR1 null would affect the control of cytokines that regulate neutrophil production and migration resulting in neutrophil influx from blood into the tissues.
Regarding function, a recent study shows that ACKR1-null allele is not deleterious to neutrophil functions and the authors propose that there is an increase in proteolytic activity, which compensates for the lower number of circulating neutrophils.9
BCN prevalence varies according to ethnic groups. An American cross-sectional study10 showed benign reductions of neutrophils in 4.5% of African-Americans, 0.79% of European-Americans and 0.38% of Mexican-Americans. A Brazilian study showed an overall frequency of 2.59% and compared to Caucasian, African, Mulatto and Asian people presented lower mean neutrophil count.3 Despite higher prevalence of BCN in Afro-descendants, self-race declared should not be used as a predictor, especially in scenarios of great admixture like Brazil.
Diagnosis of BCN is of exclusion, patients are submitted to blood tests and possibly to invasive diagnostic search until secondary causes of neutropenia are ruled out. The aim of this study is to evaluate FY phenotypes and genotypes including the analysis of the rs2814778 SNP in Brazilian patients with BCN in order to determine an effective diagnostic tool, allowing reassurance of the patient and cost reduction in their care.
Materials and methodsPatients and controlsWe conducted a case control study where a total of 95 individuals were included: 18 patients with benign constitutional neutropenia and 77 controls. The patient group was referred from Hospital Israelita Albert Einstein (HIAE) and Hemocentro of Campinas University (UNICAMP).
For the patient group, the inclusion criteria was the neutrophils count ≤2000 cells/mm3 in more than one measurement, without recurrent infections or any other cause of neutropenia and at least one year of follow-up. The patients studied do not have clinical symptoms of vitamin B12 or folic acid deficiency, inflammatory, infectious or neoplastic diseases. During the clinical investigation of neutropenia, additional tests were requested as needed in selected patients (analysis of blood smear, B12, folic acid, CRP, serology, antibodies and aspirate from bone marrow) as part of routine institutional clinical management. All patients signed an ethics consent before enrolment in this study.
The control group was formed by healthy blood donors of Hospital Albert Einstein’s Blood Bank, randomly selected. All donors signed a consent form explaining risks and benefits. The survey was conducted in accordance with the revised Declaration of Helsinki in 2008. This project was approved by HIAE’s Research Ethics Committee (CAAE: 81857318.0.1001.0071).
MethodsSerological and molecular methodsPhenotyping was performed by hemagglutination in gel cards (Grifols S.A.) adding anti-Fya and anti-Fyb (Grifols S.A., Spain, Lots: 652317022A and 652417011A).
Genotyping for FY125G > A and rs2814778 (T/C) polymorphism, were performed by laboratory developed tests (LDTs) as previously described.11
Statistical analysisPatients and controls were described by absolute count and percentual value, mean and median regarding clinical parameters of age (t-Student), gender (chi-square), and ANC (t-Student). All analyses were performed with SPSS software (version 22).
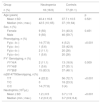
ResultsThe mean age (mean, SD) for patients and controls were, respectively 40.4 (16.8) and 37.7 (10.5) years. The groups did not differ in age or gender distribution (p > 0.05). The mean WBC count in patients were lower compared to controls (3.48 × 7.32; p < 0.001). As expected, the mean ANC count in patients were lower compared to controls (1.22 × 3.78; p < 0.001). The characteristics of the studied population are shown in Table 1.
Demographic characteristics and laboratory results of patients and controls.
| Group | Neutropenia | Controls | |
|---|---|---|---|
| n (%) | 18 (18.9) | 77 (81.1) | |
| Age (years) | p | ||
| Mean ± SD | 40.4 ± 16.8 | 37.7 ± 10.5 | 0.521 |
| Median (min.; max.) | 42.5 (10; 65) | 37 (19; 64) | |
| Sex, n (%) | |||
| Female | 9 (50) | 31 (40.3) | 0.451 |
| Male | 9 (50) | 46 (59.7) | |
| Phenotyping, n (%) | |||
| Fy(a − b−) | 14 (77.8) | 4 (5.2) | <0.001 |
| Fy(a − b+) | 1 (5.6) | 33 (42.9) | |
| Fy(a + b−) | 2 (11.1) | 20 (26) | |
| Fy(a + b+) | 1 (5.6) | 20 (26) | |
| FY* Genotyping, n (%) | |||
| FY*A/A | 2 (11.1) | 13 (16.9) | 0.009 |
| FY*A/B | 1 (5.6) | 27 (35.1) | |
| FY*B/B | 15 (83.3) | 37 (48.1) | |
| rs2814778Genotyping, n(%) | |||
| T/T | 4 (22.2) | 56 (72.7) | <0.001 |
| T/C | 0 | 18 (23.3) | |
| C/C | 14 (77.8) | 3 (4) | |
| Neutrophils (103/μL) | |||
| Mean ± SD | 1.2 ± 0.5 | 3.7 ± 1.5 | <0.001 |
| Median (min.; max.) | 1.2 (0.3; 2) | 3.7 (0.9; 6.4) | |
In the patient group 83.3% were genotyped as FY*B/FY*B, 5.6% as FY*A/FY*B and 11.1 as FY*A/FY*A in 11.1%. The SNP rs2814778 (-67T > C) was identified in 14 patients (77.8%) genotyped as FY*B/FY*B in homozygous state. As expected, all individuals with the Fy(a − b−) phenotype were genotyped as FY*B-67C/FY*B-67C. One patient with the FY*B/FY*B genotype and the remaining 3 patients with FY*A allele had the SNP rs2814778 in wild type state (T/T) enabling the expression of Fy antigens in RBCs.
In the Control Group, 3 donors (4%) were homozygous for this polymorphism (C/C), resulting in the lack of ACKR1 expression on erythrocytes (FY–), while 56 donors (72.7%) were homozygous for the wild type (T/T) and 18 donors (23.3%) were heterozygous (T/C). The neutrophil count for the 3 donors phenotyped as Fy(a − b−) and genotyped as FY*B-67C/FY*B-67C showed a mean of ± SD of 3.3 × 103/μL (±2) and none information of clinical variations was identified in the donor chart. It is a higher neutrophil mean count compared to the 14 patients that genotyped as FY*B-67C/FY*B-67C (1.08 × 103/μL ± 0.5).
The sensitivity, specificity, positive and negative predictive value were evaluated for Duffy phenotyping and genotyping. The results are shown in Table 2. Duffy genotyping showed a little better accuracy (92.6%) when compared to Duffy phenotyping (91.2%). The difference found in the characteristics of both tests was due to discrepancies between phenotyping and genotyping in a donor phenotyped as Fy(a − b−) but genotyped as FY*B-67 T/FY*B-67C. Our main hypothesis was a probably weak Fyb antigen expression (Fyx) or other genetic variant mechanism on ACKR1 resulting in the lack of Fyb expression on RBCs.
Laboratorial tests parameters and comparison between Duffy phenotyping and genotyping.
| Controls | Patients | Total | Sens | Spec | PPV | NPV | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||||
| Duffy phenotyping | |||||||||||
| Other phenotypes | 73 | 94.8 | 4 | 22.2 | 77 | 81.1 | 77.8 | 94.8 | 77.8 | 94.8 | 91.6 |
| Fy(a − b−) | 4 | 5.2 | 14 | 77.8 | 18 | 18.9 | |||||
| Total | 77 | 100 | 18 | 100 | 95 | 100 | |||||
| Duffy genotyping | |||||||||||
| Other genotypes | 74 | 96.1 | 4 | 22.2 | 78 | 82.1 | 77.8 | 96.1 | 82.4 | 94.9 | 92.6 |
| FY*B/B; rs2814778 (C/C) | 3 | 3.9 | 14 | 77.8 | 17 | 17.9 | |||||
| Total | 77 | 100 | 18 | 100 | 95 | 100 | |||||
Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: negative predictive value.
In this case-control study, we identified a strong correlation between Fy(a − b−) phenotype and FY*B–67C/FY*B–67C genotype (and the diagnosis of benign constitutional neutropenia as expected). Among the neutropenic patients, the most related phenotype was Fy(a − b−), which confirms results of previous studies.8,12FY*B–67C/FY*B–67C genotype associated with neutropenia was previously studied in Brazilians.12
The cut-off value used for the diagnosis of neutropenia is a subject of debate in the literature,13,14 including the proposal of new limits for the diagnosis of clinically relevant neutropenia.15 In the present study, an empirical cut-off value of 2000 cells/mm3 was adopted.16 Of the group of neutropenic patients, 4 did not present the Fy(a − b−) phenotype, and the neutrophil count of these individuals was 1600–1800 cells/mm3 (mean: 1700 cells/mm3).
Taking into account the objective of our study to evaluate an easy laboratory method to detect most BCN patients and our results, we could propose the use of FY phenotyping as a screening test. Although the correlation between phenotype and predictive genotype was not 100% (by the presence of heterozygotes), the individuals with Fy(a + b–) or Fy(a − b+) phenotypes did not present a low neutrophil count (and therefore would not be screened for BCN). Thus, our hypothesis is that individuals with Fy(a + b+) could, to a certain degree, rule out the possibility of BCN and Fy(a − b−) individuals should be investigated for BCN. It is relevant to note that phenotyping and genotyping are both efficient methods for detecting benign constitutional neutropenia, able to detect the majority of patients. Genotyping in our group of cases studied showed to be little bit more accurate, due to phenotyping limitations as already described in the literature,17 however additional molecular tests for the single nucleotide variants associated with weak Fyb antigen expression should be performed to confirm this hypothesis in the only phenotype/genotype discordant case.
Inconclusive diagnosis entails psychological and physical damages to the patient, besides costs to the health system. Since the ANC is low, the patient is submitted to invasive procedures, like bone marrow biopsies, chemotherapy is delayed18 and physicians may not prescribe the gold-standard treatment, as clozapine for schizophrenia.3 As observed in our study, there are donors with the predictive combination of BCN. For these cases, it is worth mentioning that the low neutrophil count is a dynamic condition and altered by several factors, such as diet and physical activity. It would be interesting to perform clinical managements to increase neutrophils or to collect blood again after physical exercise or meals.7 Thus, the use of an assertive laboratorial test allows a rational approach of the clinical assistance with adequate cost effectiveness. It also reduces the need of invasive procedures, which could lead to inconclusive results.
As other studies have revealed,12 mutation rs2814778 is more prevalent in people of African ancestry; this is why constitutional neutropenia is also known as benign ethnic neutropenia. However, although the analysis of ethnicity is relevant to the study of this condition, the Brazilian population is highly mixed, making it difficult to interpret these data objectively.
The main limitation of this study refers to the small number of samples, especially in the neutropenic patients’ group. Despite that, this study reinforces that FY phenotyping and genotyping can be used to detect BCN, avoiding excessive diagnostic investigation. Besides, this procedure may reduce health costs and be reproductible in clinical practice.
Conflicts of interestThe authors declare no conflicts of interest.
Carolina Bub, José Kutner, Nelson Hamerschlack and Lilian Castilho contributed as senior authors, aiding in the study design and conceptual ideas. Leandro Santos, Marilia Sirianni and Thiago Costa assisted in laboratory methods, project execution and scientific writing. Maria Eduarda and Mariana contributed equally in the laboratory methods, manuscript writing, design of figures and data analysis.