Cutaneous T cell lymphomas account for 2% of all lymphomas.1 Mycosis fungoides (MF) which is a low-grade lymphoma, is the most common subtype of primary cutaneous T-cell lymphomas and is closely related to its leukemic variant, Sezary syndrome. MF is also known as Alibert-Bazin syndrome or granuloma fungoides and is more commonly seen in males over the age of 50.2 The staging, classification and risk stratification of mycosis fungoides are based on the International Society for Cutaneous Lymphoma (ISCL) and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer (EORTC). Two-thirds of patients present at an early stage and will have a favourable prognosis but 25% of them may progress into disseminated advanced disease.3 In advanced stages, MF could spread to any visceral organ such as lungs, pleura, liver, spleen or lymph nodes. The lung is the most common site of extranodal involvement in MF and in most cases, lung involvement may only be known at autopsy. Prognosis for advanced-stage disease is often appalling. Here, we describe a patient with relapsed advanced mycosis fungoides with pleural involvement and unresolving pneumonia who succumbed to the disease rapidly at the intensive care unit.
Case presentationA 46-year-old male of Malay ethnicity presented to the department of hematology with a six-month history of progressive worsening of rash over the face. The rash initially commenced on the forehead and subsequently spread to the nasal bridge and nose and it was associated with bleeding. He denied any constitutional symptoms. He was known to have essential hypertension and single vessel ischaemic heart disease on which he was compliant to his medications. He had no significant family history of any malignancy, autoimmune or familial disorders. He was married with two children, a non-smoker and a teetotaller. He worked as a school-teacher.
Physical examination revealed a medium built gentleman with stable vital parameters. There was a raised ulcerating erythematous patch on his forehead extending to the nasal bridge and nose and multiple hyperpigmented lesions over the chest, abdomen and legs involving a body surface area of 45%. The largest lesion was 10 × 10 cm. He did not have any peripheral lymphadenopathy or organomegaly. The other systems were unremarkable.
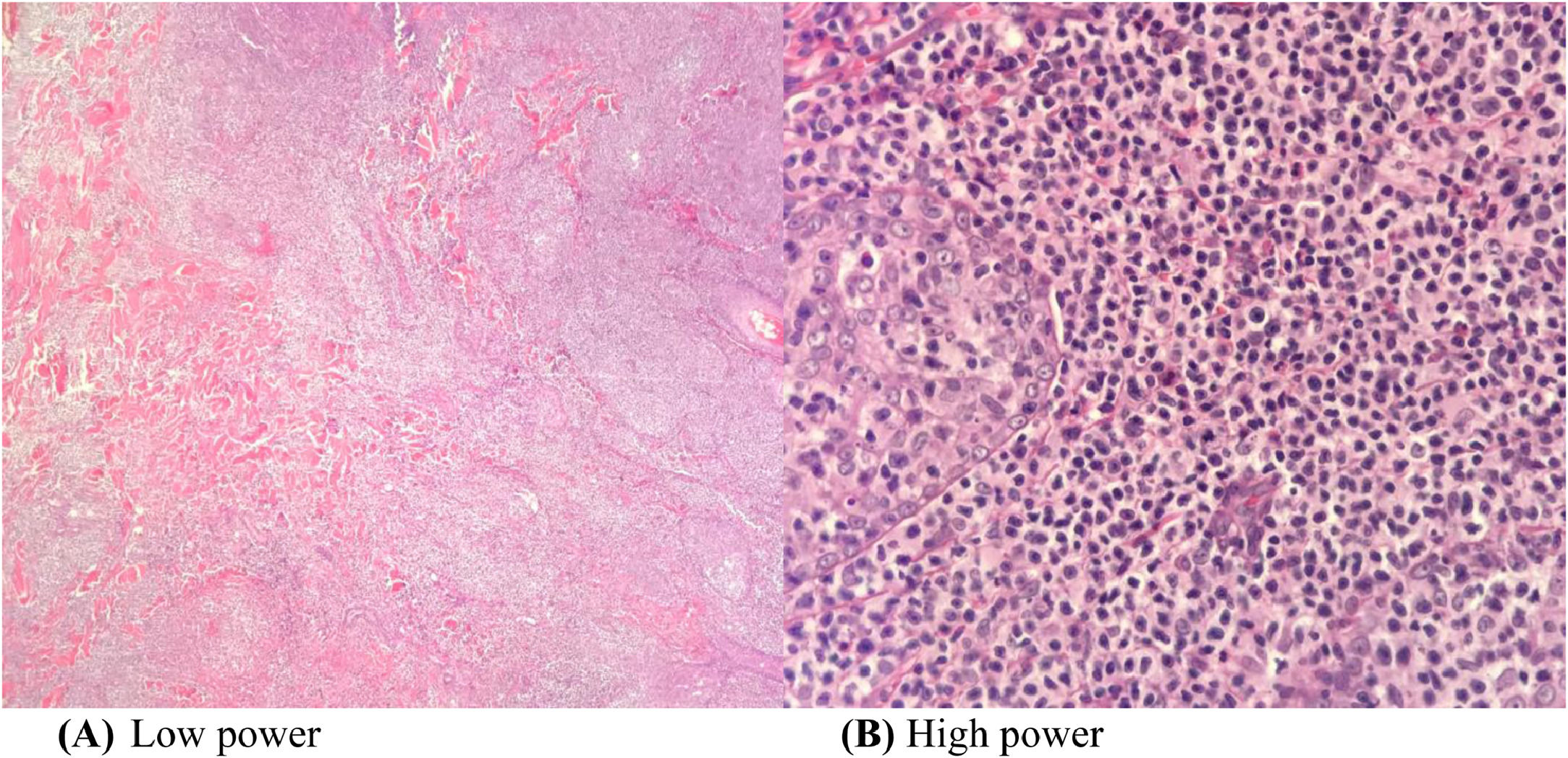
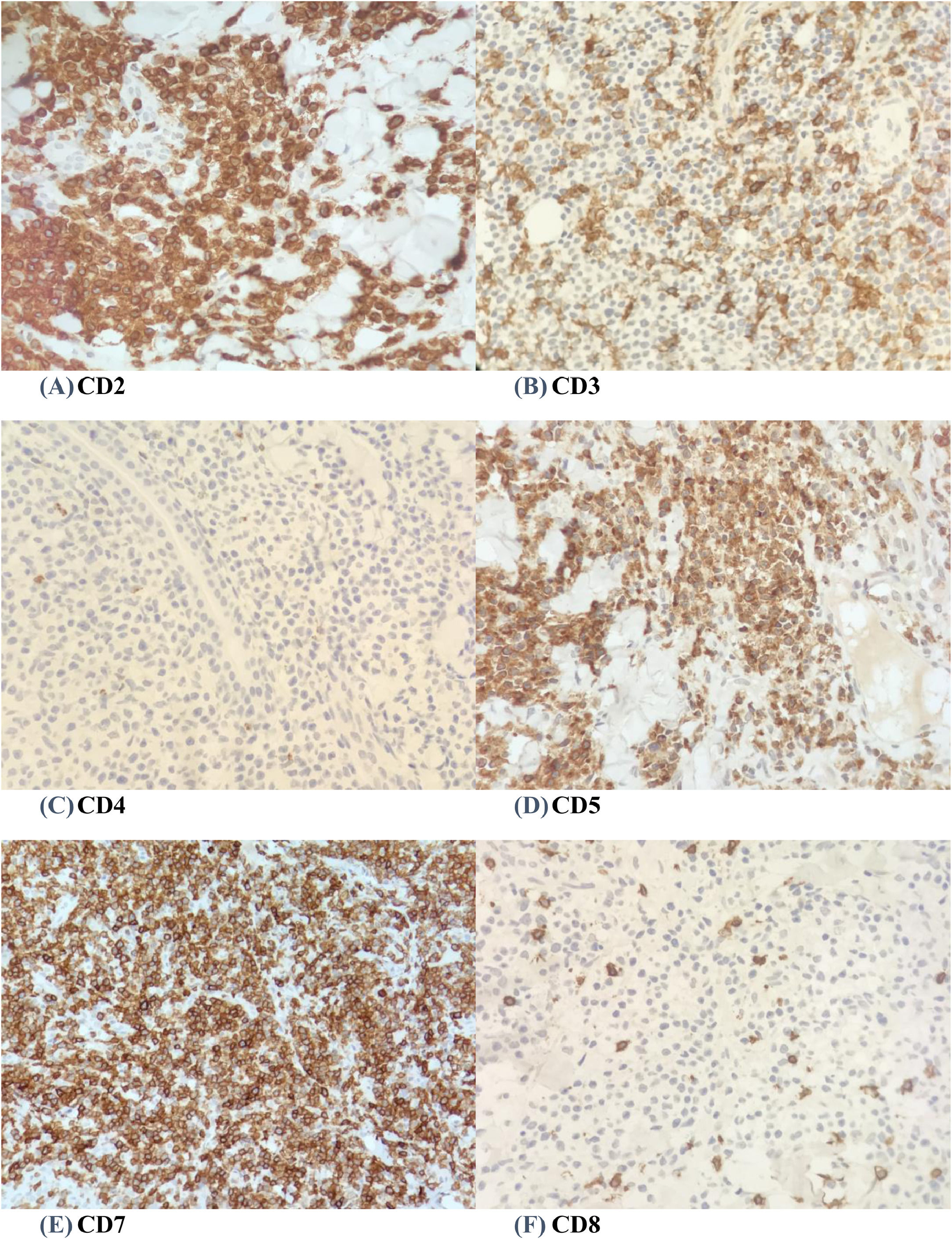
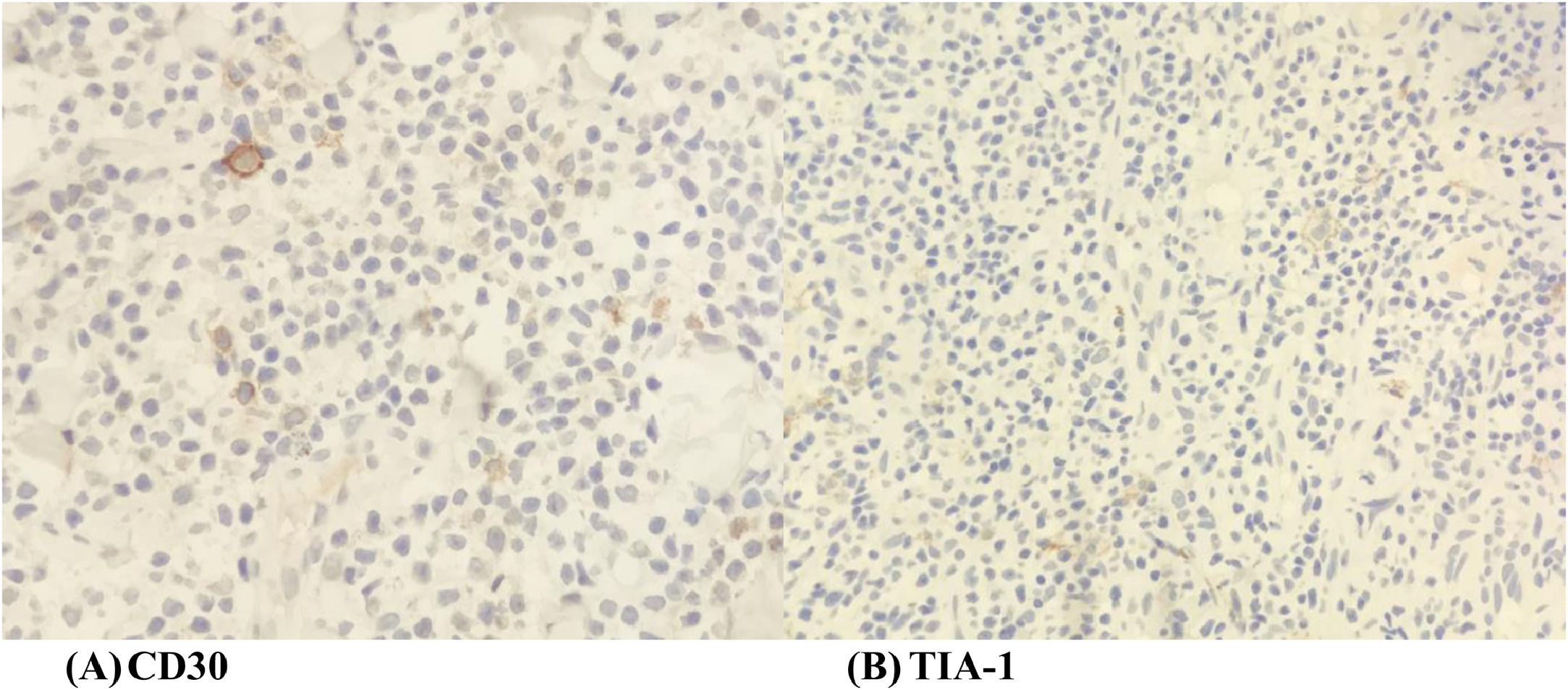
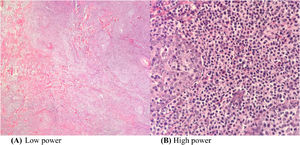
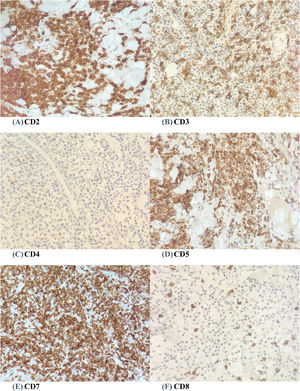
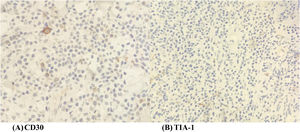
A wedge biopsy from the face showed presence of epidermotropism and malignant cells were described as diffuse small monomorphic lymphocyte infiltrates with cerebriform nuclei. There was no obvious large cell transformation on histology (Figure 1A and B). The neoplastic cells stained positive for mature T-cell phenotype markers such as CD2, CD3, CD5, CD7 and TCR-Beta with a Ki67 proliferation index of 70% (Figures 2 and 3). The cells were negative for CD4, CD8, CD20, CD26, CD30, TIA-1 and Epstein-Barr virus-encoded small RNA 1 (Figures 2 and 3). The cells were also negative for CD56, CD123, BCL11A and TCR gamma/delta. T-cell receptor (TCR) gene rearrangement studies of the formalin-fixed paraffin-embedded (FFPE) tissue by qualitative Polymerase Chain Reaction (PCR) analysis demonstrated clonal TCR-beta gene rearrangements. The overall findings were consistent with mature T-cell phenotype CD4/CD8 double-negative mycosis fungoides. The diagnostic 18-Fluorodeoxyglucose Positron Emission Tomography (18-FDG PET CT) imaging showed active lesions in the left nose, left frontal region and left lateral chest wall. Bone marrow trephine biopsy did not show any disease infiltration.
Due to the extent of the cutaneous involvement, he was treated with 20 fractions (40 Grays) of local radical radiotherapy. He was given systemic chemotherapy consisting of oral prednisolone 10mg daily, oral methotrexate 10mg weekly and subcutaneous interferon alpha 3 MU thrice weekly in which he was compliant. He did not exhibit any adverse effects of treatment.
After a year in remission, he re-presented to the department of hematology with B symptoms and dyspnoea. The previous lesions were well healed with no new skin lesions. He was progressively dyspnoeic requiring intubation for respiratory embarrassment.
The laboratory parameters are as tabulated in Table 1.
Tabulation of laboratory parameters.
The peripheral blood film did not show any abnormal lymphoid cells.
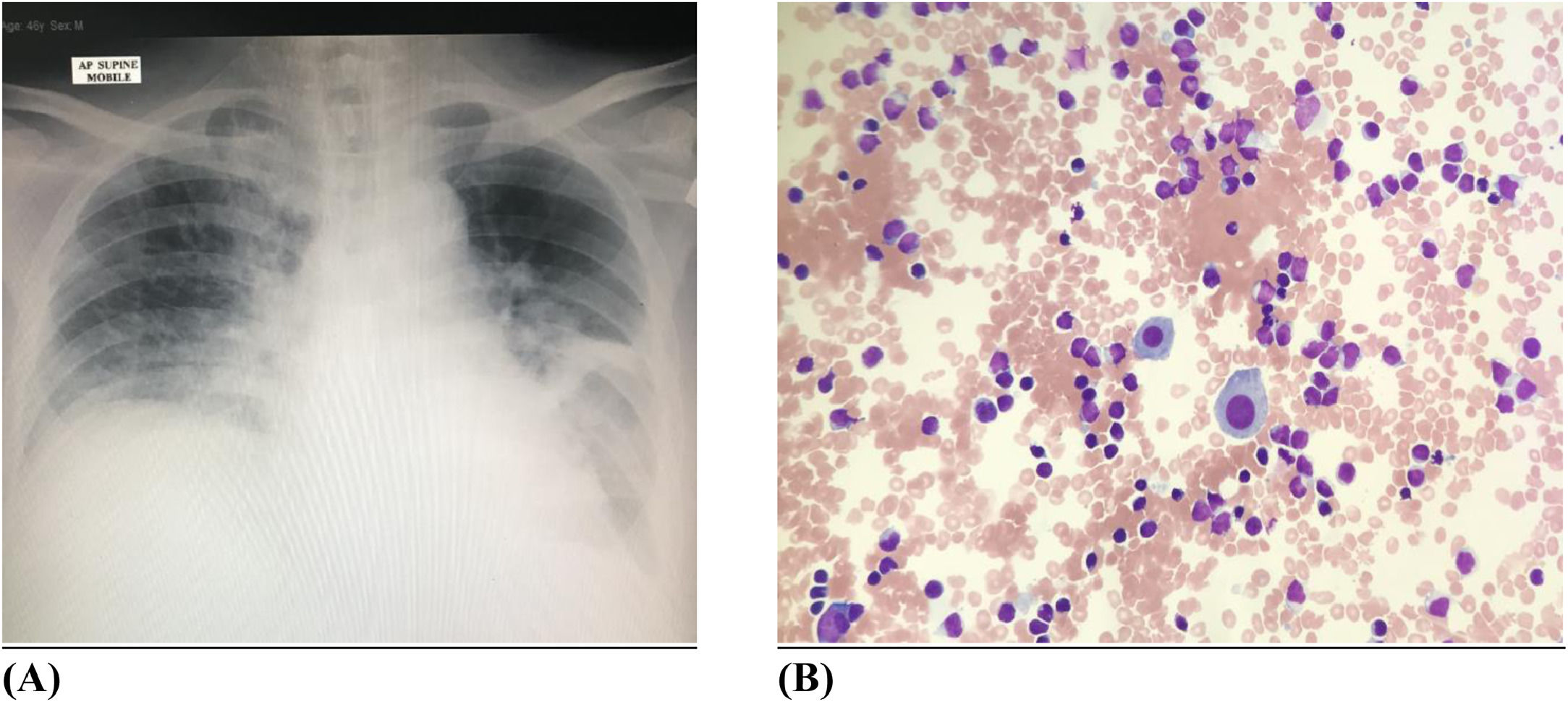
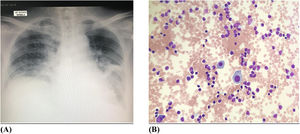
The chest radiograph (Figure 4A) showed left pleural effusion with left lung atelectasis. The whole-body CT imaging with contrast portrayed diffuse supra and infra-diaphragmatic lymphadenopathies, with the largest lymph node measuring 1.8 cm. There were nodules seen in the right upper and lower lobes of the lung measuring 1.1 cm the largest. A subsequent HRCT (high resolution computed tomography) of the thorax revealed patchy ground glass densities in bilateral lower lobes with peribronchovascular interstitial thickening consistent with lymphocytic interstitial pneumonitis.
An intercostal drain was inserted. A pleural biopsy was performed using an Abram needle. The pleural fluid was straw coloured, exudative in nature with the morphology (Figure 4B) showing abnormal lymphocytes with occasional mesothelial cells. Pleural fluid cytology and histology was consistent with neoplastic lymphocytic infiltration by mycosis fungoides. CMV inclusion bodies and fungal organisms were not seen on the Periodic Acid-Schiff (PAS) and Grocott Methenamine Siver (GMS) staining of the pleural tissue respectively. Flow cytometric analysis of the pleural fluid showed the cells were positive for CD2, CD3, CD5, CD7 and TCR-Beta. The cells were negative for CD4, CD8, CD30, CD56, TIA-1 and TCR-gamma/delta. All blood and pleural fluid cultures were sterile.
A flexible bronchoscopy was also performed at the intensive care unit which demonstrated multiple nodules in the right upper and lower lobes of the lung. The bronchoalveolar lavage (BAL) showed absence of both pneumocystic jirovecii cysts (on immunofluorescence assay) and cytomegalovirus inclusions. BAL polymerase chain reaction (PCR) was negative for mycobacterium tuberculosis. Culture of BAL fluid specimens were sterile. However, transbronchial biopsy samples were inadequate for analysis.
Autoimmune serology tests (anti-nuclear antibody, anti-extractable nuclear antigen, anti-neutrophil cytoplasmic antibodies) and peripheral blood for cytomegalovirus PCR were all negative.
He was diagnosed with relapsed disseminated mycosis fungoides with pleural involvement. He was scheduled for systemic CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisolone).
However, at the intensive care unit, he developed unresolving (culture-negative) pneumonia. Unfortunately, he succumbed to respiratory failure. It is interesting to note although MF has an indolent course, our case evolved in a little more than a year.
DiscussionWe describe a challenging case of relapsed advanced CD4/CD8 double-negative MF with diffuse lymphadenopathy, pleural involvement and recurrent chest infections. He succumbed rather rapidly at the intensive care unit suggesting the aggressive nature of the disease which is often thought to be low-grade. However, he did not manifest any leukemic phase of the disease.
The cause of MF is unknown and it is neither familial nor genetic. CD4/CD8 double-negative MF tend to occur at a younger age and was often characterised by the presence of hypopigmented-type lesions which is known to have a good prognosis.4 MF does not spread from one to another. Some clinical studies have associated MF with Human T-cell lymphotropic virus-1 (HTLV-1) but others demonstrate that HTLV-1 is not a direct causal agent of MF, thus suggesting that it may play a role in immune suppression and dissemination of the disease instead.4
Epidermotropism is characteristic in conventional MF. It is defined as atypical lymphocytes with hyperconvoluted cerebriform nuclei infiltrating the basal layer of the epidermis-stratum basale.5 Pautrier microabscesses which are clusters of four or more atypical lymphocytes in the epidermis are also an imperative feature in MF but it is largely absent.5 These similar histological characteristics are seen in CD4/CD8 double-negative MF. In typical MF, neoplastic cells originate from CD4+, CD45+/- T lymphocytes which lack markers such as CD7, CD8 and CD26. Tumour cells are often TCR-beta + and CD30-.5 Clonal T-cell receptor gene rearrangement was demonstrated in 57-71% of patients with MF and it was not associated with prognosis.5 Conventional MF usually do not express cytotoxic markers such as TIA-1 and granzyme. Some cases of CD4/CD8 double-negative MF have demonstrated expression of cytotoxic markers.
There are numerous differential diagnoses which should be considered in this patient. Among them are Sezary syndrome, primary cutaneous gamma/delta T-cell lymphoma, natural killer/T-cell lymphoma extranodal (nasal) type, primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma, lymphomatoid papulosis (LyP), blastic plasmacytoid dendritic cell neoplasm (BPDCN), primary cutaneous anaplastic T-cell lymphoma, pagetoid reticulosis, subcutaneous T-cell panniculitis and adult T-cell leukemia/lymphoma (ATLL). Sezary lymphoma frequently presents with generalised erythroderma, lymphadenopathies and palmoplantar keratoderma. Atypical T-cells (Sezary cells) are frequently seen in the peripheral blood. Our patient did not manifest any peripheral lymphocytosis or bone marrow disease. Primary cutaneous gamma/delta T-cell lymphoma exhibits red to violaceous ulcerating skin nodules in any part of the body and may give rise to hemophagocytic lymphohistiocytosis. The cells express TCR gamma and cytotoxic T-cell markers such as TIA1, perforin and granzyme B which were not present in this patient. Blastic plamacytoid dendritic cell neoplasm often presents to the clinician with skin lesions at diagnosis and may disseminate to other organ systems such as bone marrow, lungs, bone, liver and spleen. Tumour cells in BPDCN express positivity for CD4, CD56, CD123, CD303 and BCL11A on tissue immunohistochemistry. Our patient did not demonstrate presence of Epstein-Barr virus-encoded small RNA 1 which is almost always expressed in NK/T-cell lymphoma. CD8 and cytotoxic markers such as TIA-1 were negative in this patient which excludes primary cutaneous CD8+ epidermotropic cytotoxic T-cell lymphoma. This cutaneous disease frequently disseminates to the central nervous system, lungs, testis and oral mucosa. Lymphomatoid papulosis presents initially with cutaneous papules or later nodules and represents an indolent end of the spectrum of CD30+ lymphoproliferative disorders. CD30 negativity in this patient probably excludes lymphomatoid papulosis and primary cutaneous anaplastic T-cell lymphoma. In systemic ATLL, common cutaneous manifestations seen are nodulotumoral type lesions followed by plaques and patches. Primary cutaneous ATLL on the other hand may present as erythematous-papular or tumoral forms without lymphadenopathy, peripheral lymphocytosis or hypercalcemia. Both are usually related to HTLV-1 infection. However, serology testing for HTLV-1 in our patient was negative.
Lungs are frequently one of the most common internal organs of involvement in Mycosis fungoides. Many patients with advanced CTCLs succumb to recurrent lung infections due to their immunocompromised state exacerbated by poor T cell mediated immunity.6 Pneumonia is often a cause for death and the most common organisms isolated are Aspergillus fumigatus, Mycobacterium avium intracellulare and Staphylococcus aureus.6 Other opportunistic lung infections include pneumocystic jirovecii pneumonia, cytomegalovirus pneumonitis, mycobacterium tuberculosis and cryptococcus pneumonia. Radiographical pulmonary manifestations encountered in advanced Mycosis fungoides are bilateral pulmonary infiltrates, hilar lymphadenopathy, pleural effusion, parenchymal nodular densities and ground glass opacities.7 In our patient, bronchoalveolar lavage and pleural fluid studies did not yield any positive culture growth or presence of viral/fungal inclusion bodies. It is often challenging to the clinician to distinguish actual pneumonia from pulmonary/pleural involvement by MF clinically and radiographically.
The major discrepancy between pneumonia and those with pulmonary involvement by MF is that pneumonia presents with significant respiratory symptoms and imaging would depict infiltrates and consolidation.8 On the other hand, pulmonary manifestations of MF will have fewer respiratory symptoms and will show nodules on imaging which will progress gradually.8 However, both pneumonia and lung/pleural involvement by MF portray poor survival.
Management of MF is largely based on the disease stage. Topical high potency steroids (clobetasol propionate), topical nitrogen mustard or topical bexarotene (retinoids) can be used in Stage IA-IIA disease with an overall response rate of 46% and with minimal toxicity.9 Ultraviolet phototherapy (Psoralen plus ultraviolet A or ultraviolet B) and localised radiotherapy are safe and effective options in early-stage MF. Second line therapies in early-stage disease include total skin electron beam (TSEB) delivered via a linear accelerator (LINAC), oral retinoids, oral methotrexate 10-25 mg weekly and recombinant interferon-alpha 3 MU administered thrice weekly.10 A combination of oral methotrexate, subcutaneous interferon-alpha and local radiotherapy can be used effectively in early-stage MF.
In our case, since the lesions were extensive and ulcerating, we used a combination of low dose methotrexate, subcutaneous interferon-alpha and local radical radiotherapy which maintained him in remission for close to a year.
In advanced-stage disease, Stage III, combination of oral methotrexate 25 mg and extracorporeal photopheresis (ECP) is efficacious and safe with a response rate of 20-63%.11 For those in Stage IV or with Sezary syndrome, systemic polychemotherapy incorporating cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) is used but it is often ineffective.11 Other second-line treatments available include histone deacetylase inhibitors (HIDAC)-vorinostat, anti-CD30 antibody-drug conjugate such as brentuximab vedotin in CD30-positive MF and anti-CCR4 humanised monoclonal antibody such as mogamulizumab. The MAVORIC study showed significant overall response rates (28% vs 4%) and progression free survival (7.7% vs 3.1%) in the mogamulizumab arm over vorinostat.12 Allogenic stem cell transplant (Allo SCT) offers an opportunity for cure in selected patients with advanced disease.
Maintenance therapy with agents such as oral methotrexate, interferon-alpha and retinoids are important once the patient is in remission to ensure appropriate quality of life.
ConclusionMF is incurable with some patients experiencing periods of remission. Advanced disease usually results in poor survival with many suffering from intercurrent infections. Newer agents such as mogamulizumab may offer longer survival benefits in selected patients.
Ethics approval and consent to participateNot applicable
ConsentWritten informed consent was obtained from the next of kin for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Availability of data and materialNot applicable
FundingSelf-funding
Author's contributionBoth authors contributed equally to the production of this manuscript.
None.