Patients undergoing hematopoietic stem cell transplantation (HSCT) might present acute and late toxicities and the oral tissues are frequently affected. With the survival increasing, patients show late and long-term morbidities, and there is an important association between the general and the oral health. The first and second parts of this Consensus have showed the importance of the adequacy of oral health in the pre-HSCT, and the main alterations and oral care during the period of admission for HSCT. This third part aims to review specific themes of post-HSCT dental care, such as graft-versus-host disease (GVHD) and the pediatric patient. It also aims to review pertinent subjects, both during the HSCT period and post-HSCT, concerning quality of life, pain, cost-effectiveness, and remote care. Based on this review, it is evident the importance of the work of the dental surgeon (DS) in the follow-up and treatment of the HSCT patient, always collaborating with the whole multidisciplinary team.
The hematopoietic stem cell transplantation (HSCT) is a consolidation treatment for diseases where there is a deficiency and/or failure in the hematopoietic system; it is indicated to neoplastic and non-neoplastic diseases. HSCT includes a conditioning regimen with high doses of chemotherapy and/or radiotherapy for reducing cellularity in the bone marrow, followed by infusion of normal stem cells and follow-up after bone marrow engraftment.1
We must highlight that HSCT produces acute and late toxicities, and, in this regard, it is important to consider that there is an important association between the general health of HSCT patients and oral health.
In light of the world literature, a group of oral medicine experts was invited by the Brazilian Society of Cellular Therapy and Bone Marrow Transplantation (SBTMO) to prepare a guideline for dental care for HSCT patients, taking in consideration the oral health characteristics of our population.
Therefore, this is the third in a series of 3 articles that will discuss dental management in the HSCT population. These papers intend to provide continuing education to fellow dental surgeons on this specific area of care and, consequently, facilitate HSCT patient's access to dental care.
ObjectiveThis part III aims to review specific themes of post-HSCT dental care, such as graft-versus-host disease (GVHD), the pediatric patient, post-HSCT follow-up. It will also review pertinent subjects, both during the period of HSCT and post-HSCT, concerning the quality of life, pain, cost-effectiveness, and remote care.
Review methodsA narrative review was conducted on the database MEDLINE/PubMed and Embase for papers published from the period of January 2010 to December of 2020. The primary outcome was to retrieve original data on relevant articles containing dental protocols in patients undergoing the HSCT.
All original studies in English, Spanish or Portuguese assessing oral health, oral complications and dental procedures in adult and pediatric patients submitted to HSCT, were reviewed by the group of oral medicine experts. Papers written in languages other than the ones mentioned above, conference abstracts, case reports and papers including solid tumors were excluded.
The authors selected the papers that discussed dentistry and HSCT focusing on the following topics: post-HSCT dental care, graft-versus-host disease (GVHD), pediatric patient, post-HSCT follow-up, quality of life, pain, cost-effectiveness, and remote care.
The panel of experts reviewed the selected papers and then discussed the most important aspects involving oral health through virtual meetings. Thus, the recommendations in this 3-step guide were obtained based on literature information and on the clinical experience of the experts.
Review/ResultsPost-HSCT patients follow-upThe reconstitution of the recipient's immune system may take a few months after the hematopoietic stem cell transplantation (HSCT), even with an apparently normal hematologic system. Oral alterations secondary to HSCT conditioning (chemotherapy associated with total body radiation therapy or not) may cause late complications, such as dry mouth (xerostomia/hyposalivation), caries, and periodontal disease. Patients presenting reduced salivary flow and in topical corticosteroid therapy for graft-versus-host disease (GVHD) carry an increased risk of candidiasis and bacterial and viral infections in the oral cavity.1 Therefore, any dental intervention should consider the systemic condition and medications in use.1 The transplantation team must encourage patients to come to control visits to a dentist for prevention, early diagnosis, and treatment.
Oral health monitoring by a dentist should be included in post-HSCT follow-up, including regular radiographic exams, oral health advice adapted to every age range and systemic condition. Both the patients and those responsible for them should be constantly advised about preventive measures, through periodic dental prophylaxis as often as possible at every 3 to 6 months maximum, apart from the investigative clinical exam at the diagnosis of potentially malignant oral lesions and malignant lesions.2 Secondary malignancies are one of the most important late complications in post-HCT period, affecting mainly patients receiving allogeneic transplantation.3,4
Pediatric patients should have their growth and development carefully monitored due to the elevated risk of abnormalities in dental development. Patients undergoing HSCT may present gingival hyperplasia as a side effect from the use of cyclosporine and may need more frequent follow-up.2 Periodontal disease should be treated intensively until the control of the clinical picture.4
A healthy balanced diet with the reduction of the amount and frequency of sugars and acids should be recommended, especially in the cases of xerostomia/hyposalivation and treatment with topical corticosteroids or immunosuppressive drugs.5 Saliva stimulants and saliva replacement products may be necessary.
Oral herpes may become a chronic problem in this phase, even for those using acyclovir in a prophylactic dose. The use of acyclovir in therapeutic doses is effective in cases of latent virus reactivation with acute manifestations in adjusted dosage after discussion with the medical team5. In manifestations of herpes zoster infections, in other viral infections and in neutropenic patients, or in systemic treatment for GVHD, the use of an antiviral systemic treatment is mandatory.6
The literature does not bring enough information in terms of safety and efficacy of the orthodontic treatment after HSCT. The decision should take into account the individual risk of caries, the existence of hyposalivation, GVHD-secondary oral lesions, the use of immunosuppressors and topical corticosteroids. In addition, the users of antiresorptive drugs, such as bisphosphonates and anti-rank L, may present increased risk of osteonecrosis and difficulty in orthodontic movement.7 The risk/benefit of the indication of dental extraction in patients at risk of developing osteonecrosisshould be discussed in a case basis.
Patients with mouth opening limitation due to scleroderma in the face should undergo local physical therapy and have routine oral care and hygiene reinforced, once dental intervention can he hampered by access limitations.1 Mouth opening must be constantly monitored and in case of diagnosis of trismus, the patient should be referred to a physical therapist.
Dental development in children subjected to chemotherapy and total body irradiation (TBI) for HSCT might be significantly compromised. Dental development alterations are more commonly observed in children under 6 years previously subjected to intense radiation treatment. It is possible to notice altered rhizogenesis (short or V-shaped tooth roots or absence of root formation), early apical closure in 1st and 2nd molars, enamel hypoplasia, amelogenesis imperfecta, microdontia, pulp cavity enlargement, and third molar agenesis. Dental eruption appears to be unaffected by the myeloablative regimen or dental changes.8
GVHDGraft-versus-host disease (GVHD) is an immune-mediated inflammatory process, with complex pathobiology in which donor's immunocompetent T lymphocytes recognize antigens expressed by the recipient's cells as strangers, triggering an immune reaction followed by intense inflammatory responses that result in damages to patients’ different organs and tissues.9–11 Commonly affected organs are skin, gastrointestinal tract, mouth, liver, lung, eyes, genitalia, musculoskeletal system, immune and hematopoietic system, among others.9,10,12
The 2014 Consensus by the National Institutes of Health (NIH) classifies GVHD in two main categories, acute GVHD (aGVHD) and chronic GVHD (cGVHD), and recognizes that the clinical features determine whether the GVHD clinical syndrome is considered acute or chronic and not the time relationship with transplantation.12
The incidence of acute GVDH varies according to immune factors, mainly to the donor-recipient histocompatibility degree. The incidence of aGVHD, when donor is compatible and related, is about 20% to 50%, when donor is compatible but unrelated the incidence reaches 70%; and when the donor is not completely compatible and is related, it gets to 75%/80% and it is even higher when donor is unrelated, it might reach 80% to 90% of patients.9 On the other hand, chronic GVHD occurs in 30% to 70% of post- allogeneic HSCT adult patients with a growing incidence in pediatric patients as well.12,13
Oral GVHD in its acute form seems to be more uncommon with few data of incidence in the literature, whereas chronic oral GVHD shows a prevalence, ranging from 45% to 83%; in addition, the oral cavity can be the only body region affected.9,13,14
Risk factorsPrior acute GVHD and the use of stem cells from peripheral blood may be risk factors for chronic oral GVDH. However, an extensive multivariate analysis revealed that chronic oral GVDH was significantly associated with several laboratory markers of inflammation, including lower levels of albumin and higher total levels of the complement system.14
Clinical diagnosis of GVHDThe clinical manifestation of oral GVHD (Table 1) is diverse, both in its classification and in the severity of the affected tissue; it can affect any region of the oral cavity: lips, labial and buccal mucosa, tongue, hard and soft palates, floor of mouth, gingiva, as well as salivary function and restriction of mouth movement.14
Signs and symptoms of chronic oral GVHD.
Adapted from Jagasia et al., 2015.12.
Lichenoid alteration is characterized by white or milky lacy-like reticulated streaks on oral mucosa similar to Wickham's striae found in oral lichen planus, considered a diagnostic criterion for oral chronic GVHD in the setting of post-HSCT oral assessment, and it generally does not present painful symptomatology. The other clinical manifestations are insufficient to establish cGVHD diagnosis in isolation.12,14–19 However, bacterial, fungal, and viral infections, as well as potentially malignant and malignant lesions, should be considered in the differential diagnosis. 12,14,15
Patients who develop GVHD also present salivary alterations, we can observe an increase of total proteins, albumin, IgG, IL-1 and interleukin 6 (IL-6), apart from the alterations pertinent to GVHD, patients may present as well hypofunction of salivary glands and taste alterations.20 Patients who presented with GVHD showed an increase in sodium (Na) concentration and decrease of inorganic phosphorus (Pi). Calcium and magnesium do not show alterations. Therefore, the authors speculate that inorganic Na, Cl and Pi alterations might be potential biomarkers in oral inflammation.21
Salivary dysfunction can be characterized by manifestations similar to Sjögren's syndrome: hyposalivation and xerostomia. Such salivary dysfunction, in the setting of oral cGVHD, seems to be an entity different from the manifestations on mucosae, showing low correlation between both alterations. Dry mouth may cause difficulty in talking, chewing, swallowing, and it is strongly associated with the increase of diseases in the oral cavity, including caries and oral infections by Candida.14
GVHD histopathological diagnosisThe diagnostic interpretation by the pathologist requires the integration of the clinical context into microscopic alterations. The histopathological diagnosis (Table 2) represents an “instant in time” of a complex and dynamic biological process that reflects the duration, use of immunosuppressive therapy, location, and quality of the sample and histological preparation. A GVHD false-positive diagnosis may result in concomitant infections, reactions to medication or non-GVHD related inflammatory reactions; a false-negative result may also occur if the clinical correlation with the pathology is not performed.17
Histological criteria for GVHD by system or organs.
| Organ or System | Minimal criteria for acute GVHD/activity* | Specific criteria for chronic GVHD** |
|---|---|---|
| Oral mucosa/ Oropharyngeal and Conjunctiva | Lichenoid lymphocytic infiltrate with interface lesion, exocytosis, and variable apoptosis. † | |
| Minor salivary gland | Periductal lymphocytic infiltrate with infiltration and damage of intralobular ducts, fibroplasia in periductal stroma, lymphocytic and plasmocytic inflammatory infiltrate with destruction of acinar tissue.‡ |
†Inflammation of oral mucosa and within minor salivary glands may persist due to chemoradiation or prior inflammation. The distinction between acute and chronic GVHD requires the addition of distinct oral manifestations.
Adapted from Shulman et al., 2015.17.
The distinction between acinar destruction and previous fibrosis and ongoing chronic GVHD activity can be difficult, and it depends on the evaluation of lobes that are not completely fibrotic. Acinar and periductal inflammation with characteristics of damages to ducts, such as vacuolar alteration, lymphocytic exocytosis, nuclear dropout, dyspolarity or apoptosis, and resulting fibroplasia indicate chronic GVHD activity.
The treatment of oral acute and chronic GVHD aims at the recovery of mucosa integrity and, in consequence, the improvement of patients’ quality of life. In the contemporary therapeutic arsenal, we can use systemic immunosuppression mainly in refractory cases or cases difficult to control, associated or not with topical treatment. We should consider that the use of topical corticosteroids increases the risk of oral, viral, or fungal infections. The adequacy of the oral cavity performed through guidance on oral hygiene good practices and intervention is fundamental in all periods related to HSCT and it is considered an important part of the treatment.14,18,19
In the last five years, new treatments for chronic GVHD with oral involvement not were published. We can mention the systemic treatments that enabled partial responses of chronic GVHD in patients who also had oral mucosa involvement, such as Tocilizumab,20 Ruxolitinib.22,23 As for topical treatments for oral chronic GVHD associated or not with systemic treatment, we can cite some publications of a series of cases using platelet-rich fibrin – PRF2,24 and still contradictory use of low power laser,25–27 with satisfactory results, but with low scientific evidence. Patients with symptomatic oral GVHD may need topical treatment for two years or longer, steroid therapies and tacrolimus are safe and effective in this management, however second line treatments for refractory oral GVHD require further investigation.28–30
Post-HSCT patients who had oral chronic GVHD for a long period have higher risk of developing a second primary cancer in the oral cavity, particularly squamous cell carcinoma.31 Carriers of Fanconi anemia (FA) show 700 to 800 times fold chances of developing malignant alterations when compared with general population.32 Squamous cell carcinoma (SCC) in the head and neck region is among the most frequent carcinomas in these patients.4 Early diagnosis in these individuals is essential and ensures a better prognosis. Therefore, cGVHD carriers and FA patients demand differentiated dental follow-up.1 Control dental visits should be individualized according to patients’ clinical history and their needs, but in patients without complaints and/or lesions in the oral cavity, appointments can be rearranged at every 6 months after HSCT. For patients diagnosed with Fanconi anemia, dental assessment is mandatory at most at every six months.31 Options of support treatment for oral GVHD are described in Chart 1.
Support therapy for oral GVHD treatment.
| Situation for treatment Indication | Presentation | Medication | Concentration | Grade of recommendation |
|---|---|---|---|---|
| Lichenoid lesions / erosions /ulcers | Mouthwash | ClobetasolBudesonideDexamethasoneTriamcinolone TacrolimusPrednisoloneClobetasol:tacrolimus 1:1 | 0.5 mg/mL (0.05%)0.3 mg/mL (0.03%)0.1 mg/mL (0.01%) 1 mg/mL (0.1%) 0.1 mg/mL (0.01%) 3 mg/mL (0.03%) Not specified | AIaBIIaAIIIAIIIBIIaBIIICIII |
| Gel, paste, ointment | Clobetasol gel Tacrolimus ointmentFluocinonide gel | 0.05% 0.1% 0.05% | AIaBIIaBIII | |
| Intralesional injection | Triamcinolone | 40 mg/mL;0.5 mL/cm2 | CIIb | |
| Pain in oral mucosa | Mouthwash | LidocaineKaolin-pectin-diphenhydramine-lidocaine 1:1:1 | 2% Not specified | BIIIBIII |
| Not applicable | Low power laser (infrared) for pain relief | Not applicable | CIII | |
| Xerostomia/Hyposalivation | Toothpaste/ Gel/ liquid | Fluoride | According to manufacturer | AIb |
| Liquid | Sipping water | Whenever necessary | AIII | |
| Chewing gum | Salivary Stimulants | Whenever necessary | AIII | |
| Gel or spray | Oral mucosa lubricant | Whenever necessary | BII | |
| Tablets | Pilocarpine cevimeline | 5–10 mg X 3–4/d 15–30 mg X 3/d | BIIaBIII |
Adapted from Carpenter et al., 2015.33.
Antineoplastic treatment applied to children, especially in the pre-HSCT period, such as total body irradiation therapy (TBI) and/or chemotherapy, may result in late alterations in dental development. Permanent dentition starts its development with intraosseous calcification of the first permanent molar between 1 and 4 years and ends around 18 years with the mineralization of the third molar. Most pediatric patients subjected to HSCT are at this age range. Therefore, potential alterations in craniofacial development and dental eruption have a direct relationship with the age range and treatment employed.34,35
The most common dental alterations in children subjected to onco-hematologic treatment before 6 years old are: taurodontism, macrodontia, supernumerary roots, supernumerary teeth, microdontia, root dilaceration, root shortening and ectopic tooth eruption. For the diagnosis of these abnormalities, the gold-standard imaging exam is the panoramic radiography associated with physical examination by the dental surgeon.5,34
As for dentition alterations, craniofacial growth and development may be affected in this group of patients. Alterations in the standard of craniofacial development with lower vertical growth of the face have been discussed in the literature.36–38
Possibilities and limitations of orthopedic and orthodontic movement were not stressed in the literature. Immunosuppressants used in HSCT may interfere directly in the inflammation and bone remodeling.39 The bone remodeling and the relationship with drugs used to prevent GVHD in adults show that they are at risk of osteoporosis secondary to bone loss associated with their underlying illness, particularly in female.40 Orthodontic treatment should be simplified and individualized considering the limitations of each case.41
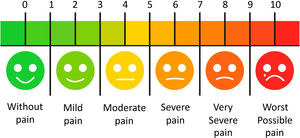
Quality of life and painIt is known that pain has a direct impact on the individual's quality of life. In HSCT, the oral cavity is a site for several manifestations of toxicity that generate painful symptomatology, the main conditions are oral mucositis and GVHD. Therefore, using criteria for assessing pain grading and how much these conditions affect the quality of life is paramount to establish properly diagnoses and treatments. The Visual Analogue Scale (VAS)42 (Figure 1) of pain can be used for assessing adult and pediatric patients during and after HSCT.
Visual Analogue Scale, adapted from Phan et al., 2012.42
For the assessment of the impact of oral health condition on the quality of life (QoL) and also the assessment of overall quality of life, questionnaires as OHIP-14, FACT-G and QLCOH15,43–46 FACT-BMT; SF-36,47 PROMIS; PROMIS-29 and Lee Symptom Scale48 have been used. In terms of pain, the questionnaires WHOOTS and MTS46,49 were found in the literature which have already been proposed and used during the transplantation period, assessing functional limitations when oral mucositis or oral GVHD was present.
Among the questionnaires mentioned in the literature, FACT-BMT for the assessment of overall quality of life and OHIP-141 for the assessment of the impact of oral health on the quality of life are the most used during HSCT. Studies have shown that the analysis of these results have modified treatment strategies, sometimes changing the concept of therapeutic efficacy and efficiency. In light of the above, incorporating this type of tool capable of measuring pain and QoL in patient care is fundamental, contributing to more humanized care.
Cost-benefit and cost-effectivenessThe importance and the impact of the dental management of the patient undergoing HSCT have enabled a growing integration of the Dentist into multidisciplinary teams. It is up to the Dentist to establish clinical protocols dedicated to prevention, diagnosis, and treatment of manifestations of toxicity in the oral cavity, collaborating to reduce the risk of infection and to improve these patients’ quality of life5.
Studies have shown that the removal of oral infection foci before the patient starting the HSCT process, oral care, and protocols of photobiomodulation (lasertherapy), performed during the conditioning period until bone marrow engraftment, result in a significant reduction, up to 13 times, the extension and severity of OM and, in consequence, there is a decrease in its clinical ramifications, as shown in .50
This reduction of comorbidities resulting from OM impacted the time of admission of the patient during HSCT, decreasing in up to 5 days when the dental care protocol was applied. The costs for including the dentist and photobiomodulation were also measured and even so the result was an impact of about 30% on the reduction of HSCT general costs.50
SurvivalHematopoietic cell transplantation (HSCT) is a consolidation treatment for diseases in which there is a failure and/or deficiency of the hematopoietic system, including both neoplastic and non-neoplastic disorders. HSCT procedure includes the reduction of bone marrow cellularity by means of conditioning with high doses of chemotherapy and/or radiotherapy, followed by an infusion with normal stem cells. The stem cells will replace the original hematopoietic cells precursors, improving the free-disease survival. The study evaluated the development of OM in all the patients exposed to PBMT and the impact of this condition on the overall survival and concluded that moderate and severe OM affected the overall survival of these patients.51
Remote careThe World Health Organization defined telemedicine as “The delivery of health care services, where distance is a critical factor, by all health care professionals using information and communication technologies for the exchange of valid information for diagnosis, treatment and prevention of disease and injuries, research and evaluation, and for the continuing education of health care providers, all in the interests of advancing the health of individuals and their communities.” World Health Organization (WHO).52
In the field of HSCT and in support to late oral manifestations of transplantation, sometimes teledentistry will be the fastest and agilest resource for communication between patient and dentist, where information of imaging, results of laboratory tests, and symptoms might lead to the final diagnosis onsite, accelerating the application of the appropriate treatment.
Teledentistry offers recommendations for the future and sheds light on the different types of teledentistry and how it can be incorporated into the practices by the regulatory authorities in Brazil.
ConclusionAs shown in this consensus, the dentist play a great role in the treatment of the patient subjected to HSCT, collaborating with the multidisciplinary team in the maximization of the results of this therapy.
Statement of clinical relevanceThe complications in the oral cavity may increase mortality and morbidity in the different phases of the hematopoietic stem cell transplantation. There is not a Brazilian guideline that guides the work of dentists with patients subjected to HSCT. This third part of the consensus offers guidance towards post-HSCT dental intervention.