In patients with a subjacent malignancy, the development of non-infectious granulomas at sites where there is no evidence of malignant involvement is a well-recognized phenomenon but the participation of the skin is rare.
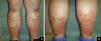
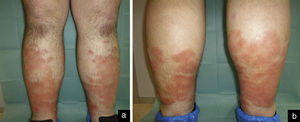
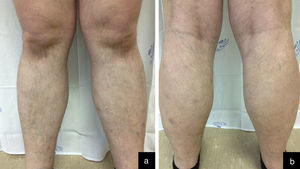
Case reportA 33-year-old male was referred to our department with a three-month history of weakness, nonproductive cough and asymptomatic skin lesions of both legs. His medical history included asthma that was medicated with montelukast, budesonide and formoterol. A physical examination showed confluent erythematous annular plaques, circumferentially distributed on both legs and associated with edema (Figure 1). No hepatomegaly, splenomegaly nor lymphadenopathy were palpable.
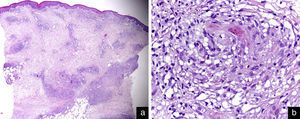
A biopsy specimen of a cutaneous plaque revealed a lymphohistiocytic infiltrate with a non-necrotizing granulomatous pattern involving the dermis and subcutaneous tissue (Figure 2). No atypical cells or the presence of foreign bodies was observed. Tests for bacteria, acid-fast organisms and fungi were negative. Bacterial, fungal and mycobacterial cultures of the skin and polymerase chain reaction to detect Mycobacterium tuberculosis, Candida albicans and Aspergillus fumigatus in cutaneous specimens were all negative.
Histopathological findings. (a) Lymphohistiocytic infiltrate with a non-necrotizing granulomatous pattern involving the dermis and subcutaneous tissue (hematoxylin–eosin stain: original magnification ×20). (b) Detail of a cutaneous granuloma (hematoxylin–eosin stain: original magnification ×400).
Laboratory tests showed mild anemia (hemoglobin 12.4g/dL; normal range: 13.0–18.0), relative neutropenia (14.8%; normal range: 53.8–69.8%) and lymphocytosis (71.8%; normal range: 25.3–47.3%) and an increased angiotensin-converting enzyme level (81U/L; normal range: <52U/L). Renal and hepatic profiles, and serum and urinary calcium levels were all normal. Serological tests for HIV, hepatitis B and C virus, syphilis and Lyme disease were negative.
In order to exclude systemic sarcoidosis, a chest radiography, ophthalmologic examination, lung function tests and electrocardiogram were performed none of which identified any relevant abnormality.
The patient also underwent gallium scintigraphy, which revealed increased uptake of the radioactive agent in the right lower quadrant of the abdomen, suggestive of retroperitoneal adenopathic conglomerates. Excisional biopsy of an abdominal lymph node was performed. Microscopic examination showed a polymorphous infiltrate of lymphocytes, histiocytes, eosinophils and large atypical cells with the appearance of Reed-Sternberg cells in ill-defined nodules separated by fibrous bands. Immunohistochemical stains showed that large atypical cells were positive for CD30 and negative for CD45, CD20, CD3, CD15 and CD68. Small lymphocytes were mainly T cells (CD3+), although some B cells (CD20+) were also observed. Immunostaining of CD68 confirmed the presence of an abundant population of macrophagic cells. The diagnosis of classical Hodgkin's lymphoma, nodular sclerosing subtype, was established.
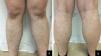
After staging, the patient began an adriamycin, bleomycin, vinblastine, dacarbazine (ABVD) chemotherapy regimen. Partial remission of the hematologic malignancy and regression of the cutaneous lesions were observed after four cycles of treatment (Figure 3).
DiscussionIt is well known that patients with malignant tumors develop non-infectious granulomas in non-involved organs.1,2 Lymph nodes, the liver and spleen are the organs most frequently affected with skin involvement being a rare phenomenon.
In 1986, Brincker described the occurrence of sarcoid reactions in 4.4% of carcinomas, in 13.8% of patients with Hodgkin's disease, and in 7.3% of cases of non-Hodgkin lymphomas.3 He claimed an association between sarcoidosis and malignant lymphoproliferative disease and proposed the existence of a sarcoidosis-lymphoma syndrome.3 The term “sarcoidosis-lymphoma” however, is not always appropriate as many patients only exhibit sarcoid-like tissue reactions with clinical or laboratory evidence insufficient for diagnosing sarcoidosis.4,5 In fact, in the case we present, despite the presence of cutaneous sarcoid-type granulomas and an elevated angiotensin-converting enzyme, no other systemic manifestations of sarcoidosis were detected.
Although the few reported cutaneous histologic features are mostly those of sarcoid-type non-caseating granulomas, other features, such as tuberculoid and palisaded granulomas, have been described.6
The exact cause of granulomatous reactions in systemic lymphomas needs to be clarified. Some authors consider that these reactions are caused by antigenic factors derived from the tumor cells.1,7 Others suggest mechanisms that include reactions to foreign bodies or against unidentified opportunistic microorganisms, or reactions to chemotherapy or to the contrast media used in tumor staging.4,8 In our patient, neither the presence of foreign bodies nor opportunistic infections were identified, and, in the lack of a temporal correlation between the onset of skin lesions and chemotherapy or imaging tests, there is no support as to a triggering role of these factors.
Mainly due to the paucity of published cases, the prognostic implications of cutaneous granulomas in the setting of systemic malignancies remain unclear. It has been hypothesized that, as they serve as a host-protective response against the tumor, the granulomas would be a good prognostic indicator, although, some cases describing an aggressive course of the disease, contradict this claim.4,9
We describe a patient who presented with a non-necrotizing granulomatous skin reaction, without evidence of malignancy, and, in whom the complementary study diagnosed Hodgkin's lymphoma. Other causes of cutaneous granulomas were excluded. Chemotherapy led to partial remission of the tumor and resolution of cutaneous lesions, suggesting a narrow relationship between the skin reaction and systemic malignancy. We stress that the possibility of systemic lymphoma should be considered in patients presenting with non-infectious granulomatous skin reactions in the absence of a better explanation.
Conflicts of interestThe authors declare no conflicts of interest.