The neoplasm of plasma cells is a group of diseases encompassing from monoclonal gammopathy of undetermined significance (MGUS) to multiple myeloma (MM).1
Conglomerates of monoclonal plasma cells are called plasmacytomas and are included in this group.2 They can occur in isolation or associated with MM, with the most common location being bone tissue.3
The existence of extramedullary plasmacytomas has also been described. Most commonly, they affect the head, neck and subcutaneous tissues, especially of the upper respiratory tract.4
They are present in 7–19% of patients at the time MM is diagnosed and in another 6–20% during the course of the disease.5
Breast plasmacytoma is a rare condition. In 2006, Taylor et al.,2 described 43 cases in the literature. Diagnosis is made by pathology however imaging can further aid the diagnosis of the disease. Ultrasound and mammographic aspects have already been described in the literature, as have aspects of magnetic resonance imaging.2–4 A case of MM with mammary gland involvement at diagnosis is described herein.
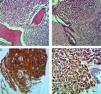
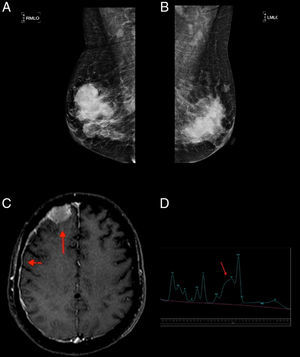
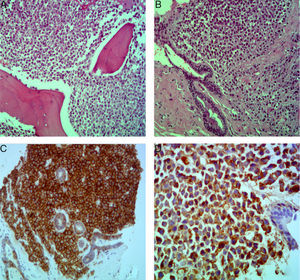
Case reportA 52-year-old, Black female patient was admitted to hospital with asthenia. She had suffered 10kg of weight loss, pain in the thoracic region and vomiting. The physical examination showed pain on palpating the chest and bilateral breast lumps. Initial tests showed bicytopenia (anemia and thrombocytopenia), renal dysfunction, and hypercalcemia. First, the symptomatic hypercalcemia was controlled and, subsequently, an investigation into the etiology was carried out, with mammary neoplasm being the initial hypothesis. Mammography showed hyperdense nodules, with regular margins and lobulated contours, distributed bilaterally (Breast Imaging-Reporting and Data System – BI-RADS 0), whereas ultrasonography showed multiple solid oval-shaped nodules with lobulated contours and with vascularization by color Doppler (Figure 1). A core biopsy was performed. Test results, however, ruled out primary breast cancer while showing breast plasmacytoma, with lambda chain restriction, plasma cell ratio of 95%, CD138+ and negative for CD56 (Figure 2). Other tests conducted to confirm MM were positive. Protein electrophoresis identified monoclonal components (5.34g/dL), immunofixation identified IgA/Lambda and bone marrow biopsy showed bone marrow plasmacytosis 90%, CD138+, and Lambda+ (Figure 2). Hence, a diagnosis of IgA/lambda MM with extramedullary plasmacytoma located in the breast was reached, with an international staging system score of III and Durie Salmon (DS) stage of IIIB.
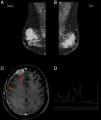
(A and B) Mammography: hyperdense and lobulated nodules with circumscribed margins; (C) extra-axial expansive lesion compatible with plasmocytoma in the right frontal convexity (arrow) with pachymeningeal impregnation (arrowhead), demonstrating central nervous system involvement; (D) spectroscopy of the same patient showing an increased glutamine–glutamate complex (arrow) resulting from elevated central nervous system ammonia.
The patient underwent induction chemotherapy with the administration of the CTD regimen (cyclophosphamide, thalidomide, and dexamethasone). After three cycles, her clinical condition worsened with altered level of consciousness, seizures, and disease progression with new plasmacytomas (sternum and central nervous system−CNS) and hyperammonemia being observed. This metabolic change was established by measuring the serum ammonia level (93μmol/L; normal value up to 32μmol/L) and confirmed by proton magnetic resonance spectroscopy (PMRS), which showed a glutamine/glutamate peak and reduced choline and myo-inositol (Figure 1).
The chemotherapy regimen was then changed to a combination of dexamethasone, thalidomide, doxorubicin, cisplatin, cyclophosphamide, and etoposide (DT-PACE). After three cycles of this chemotherapeutic regimen, the monocolonal component reduced completely and there was a reduction in the extramedullary plasmacytomas (breast, sternum, and CNS). Treatment then proceeded with high-dose melphalan and rescue with autologous hematopoietic progenitor stem cell transplantation and subsequent radiotherapy against the sternal plasmocytoma.
Disease progression was observed 30 days after the transplant with meningeal infiltration, with the liquor having 88% of plasma cells positive for CD38, CD56, CD117, CD138, and CD200. The patient underwent palliative treatment with intrathecal chemotherapy (methotrexate, cytarabine, and dexamethasone).
She evolved to death ten months after diagnosis, with multiple extramedullary plasmacytomas (breast, sternum, CNS, subcutaneous tissue) and absence of any monoclonal component.
DiscussionExtramedullary plasmacytomas of the breast are rare; they have been found at diagnosis in only 14% of cases. Most (75%) plasmacytomas of the breast occur during relapse of MM.2
The epidemiological characteristics of the case described herein are consistent with the data in the literature, regarding gender and age. Most breast plasmocytomas occur in women with a mean age of 53 years at diagnosis, but predominantly unilateral.2,3
The mammographic results of this patient are in agreement with the findings reported in the literature as well-defined masses with irregular margins. On the other hand, the ultrasonographic pattern described by authors is heterogeneous, with hyperechoic, hypoechoic, or anechoic lesions being reported.3
The prognosis of patients with extramedullary plasmacytoma is variable. Solitary plasmacytomas have a good prognosis (radiotherapy and surgery are curative treatment options) and less than 30% of cases develop MM or other plasmacytomas, whereas the disease progresses with unfavorable outcomes in patients with MM and extramedullary primary or secondary plasmacytomas, as in this case.5
Progression of the disease was observed in the current case with new extramedullary plasmacytomas despite of the CTD regimen. Using PMRS and determination of serum ammonia levels, hyperammonemic encephalopathy was also found. This condition together with MM and without liver disease is rare (3.8%). Nevertheless, it should be considered in patients with altered levels of consciousness, as observed in this patient.6,7
Hyperammonemia was not considered as the only single factor for the altered mental status, but part of a complex scenario, which included hyperviscosity of the blood, CNS infiltration, and hypercalcemia.
This encephalopathy is caused by the production of ammonia at critical levels by the plasma cells themselves, thereby exceeding the depuration capacity of hepatocytes.8
In the systematic review by Pham et al.,6 hyperammonemic encephalopathy was more commonly related to myeloma subtypes IgA and IgG, advanced stages of the disease, and CNS infiltration, which leads to a worse prognosis with higher rates of hospitalization and mortality.9,10
Considering the progression and aggressiveness of the disease, the choice was to modify treatment to the DT-PACE regimen.11 Bladè et al.12 found that extramedullary disease appears to be more properly treated with intensive chemotherapy regimens, including new agents such as lenalidomide and pomalidomide, compared to traditional therapies for MM; however, this option is not clearly described in the literature yet.
Extramedullary disease, whether the patient has plasmacytomas of the bone or not, may be the most important clinical finding in the course of MM, as in this case. The biological mechanisms underlying this occurrence have not been properly established, but a series of events are postulated. These including decreased expression of adhesion molecules (CD44 and CD56), increased angiogenesis, loss of cytokine receptors responsible for plasma cell homing [C-X-C chemokine receptor type 4 (CXCR4) and C-X-C motif chemokine 12 (CXCL12)] and bone marrow hypoxemia, causing these cells to migrate to the periphery. Although the loss of CD56 expression facilitates hematogenous or bone dissemination, its expression is paradoxically associated with impairment of specific sites, such as the CNS.13,14
The heterogeneity in CD56 marking could be duly evidenced in this case, where there was loss of CD56 expression in the plasmocytoma of the breast and, during the patient's progression, this marker was shown to be positive when immunophenotyping the liquor; this suggests the influence of as yet unknown mechanisms and other factors.
Although cytogenetics assays were not carried out, this does not seem to be a finding capable of predicting outcomes. Varga et al.,15 in a study of 117 patients, demonstrated that unfavorable cytogenetics results at diagnosis did not determine the occurrence of extramedullary disease or poor prognosis.
The clinical characteristics and laboratory results of this patient were already indicative of a poor prognosis with low chances of survival at diagnosis (ISS: III). Her progression with extramedullary plasmocytomas, probably due to hematogenous dissemination, only corroborated the biological complexity of the disease. The median survival time reported for these cases is ten months in spite of the treatment administered, as was seen in this case.13,15
Conflict of interestThe authors declare no conflicts of interest.