Non-Hodgkin lymphomas (NHL) are a heterogeneous group of lymphoproliferative disorders originating in lymphocytes or natural killer cells with most cases being B-cell lymphomas. The typical presentation is of a rapidly enlarging tumor mass at single or multiple nodal sites although up to 40% of patients may have disease initially confined to extranodal structures.1
Inflammatory breast carcinoma (IBC) is a rare and particularly aggressive form of breast cancer, characterized by rapid onset of erythema and edema (peau d’orange) occupying at least one-third of the breast. The diagnosis is made on clinical grounds associated with pathological documentation of invasive carcinoma in the breast parenchyma.2,3
We describe a female patient with an enlarged, erythemic and edematous left breast, regional adenopathy and poorly differentiated carcinoma on hematoxylin and eosin (H&E) staining, who after proper imaging and immunohistochemistry proved to have a high-grade axillary B-cell NHL presenting under the guise of IBC.
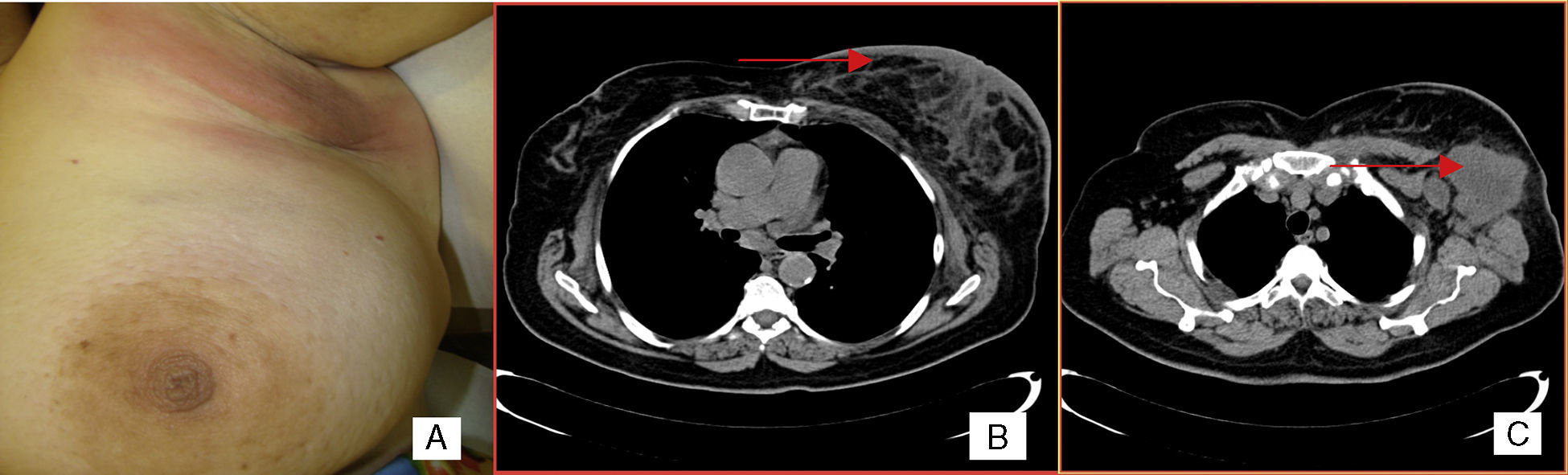
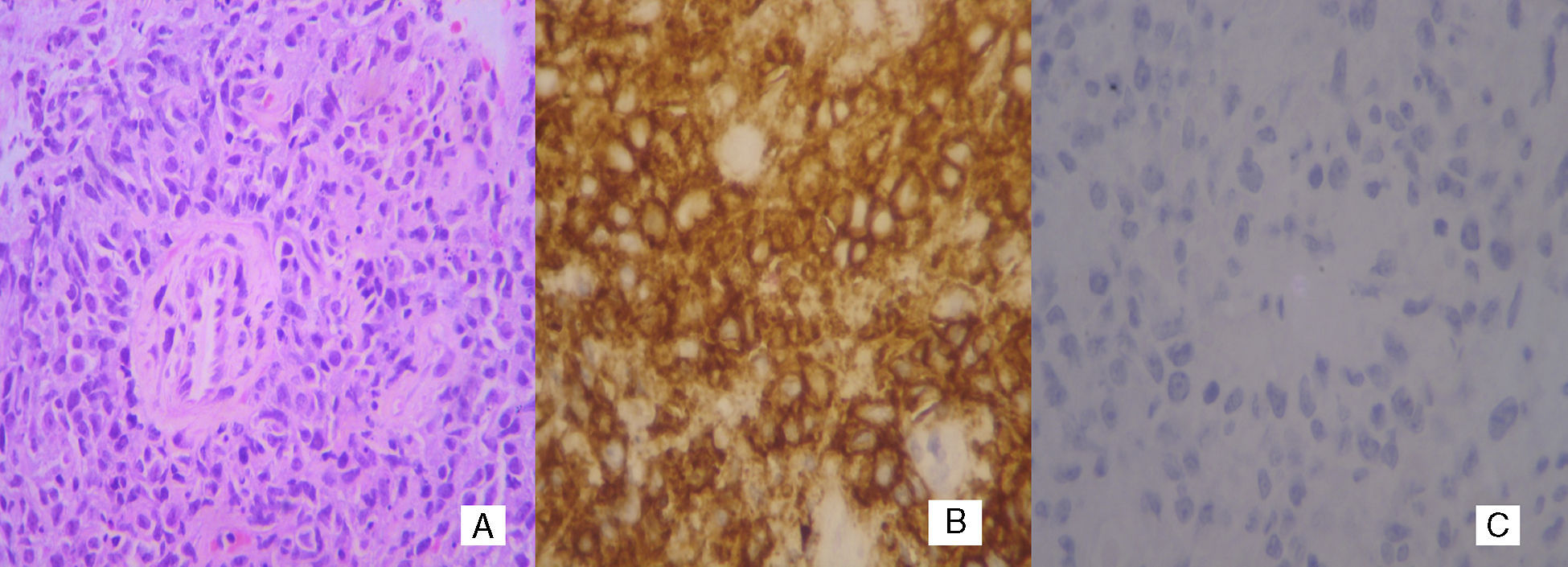
Case reportA 66-year-old woman presented with a 3-month history of left breast enlargement, erythema and peau d’orange skin edema (Figure 1). There was no palpable nodule or nipple discharge. The erythema extended into the left axilla, where a voluminous, ill-defined mass was identified. No other masses or lesions of the right breast or elsewhere were noted on the exam. Bilateral mammography and breast ultrasound showed abnormalities of the left breast: skin thickening, diffusely hyperechogenic breast parenchyma, dilated intramammary lymphatics and a hypoechogenic mass (extensive adenopathy) in the left axilla; no nodules or microcalcifications were apparent. Such findings were also apparent on a chest computed tomography (CT) scan (Figure 1). Incisional biopsy of the axillary mass was carried out. H&E sections revealed subcutaneous infiltration by poorly differentiated carcinoma (Figure 2). Given the changes of the left breast reminiscent of IBC, punch biopsy of the breast skin was performed. Results showed dermal angiolymphatic ectasia and benign perivascular lymphocytes; a deep core biopsy of the breast parenchyma showed similar histologic features with no neoplastic cells. The immunohistochemistry panel (CD20+ diffuse; CD3− Ki-67+ in 80% of cells; negativity for the epithelial antigens AE1/AE3, estrogen receptor, progesterone receptor, Her2-neu) supported the diagnosis of high-grade B-Cell NHL (Figure 2). Significant constitutional symptoms were absent. Staging procedures (chest and abdominopelvic CT scans, positron emission-computed tomography (PET-CT), cerebrospinal fluid analysis, bone marrow smear and biopsy) revealed stage IVA, high-risk international prognostic index (IPI) 4 disease, with mediastinal adenopathies, matted lymph nodes forming a 6.8cm mass involving the adipose tissue of the left axilla, and multiple secondary lung and splenic nodules. The patient was treated with eight cycles of systemic chemotherapy (R-CHOP-21: rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone), cycling every 21 days, and central nervous system prophylaxis with intrathecal methotrexate. The treatment was well tolerated, resulting in complete resolution of breast abnormalities and systemic disease control on interval CT scans. PET-CT at the end of chemotherapy showed the disease had disappeared from all sites apart from a residual, involuted and metabolically faint left axillary adenopathy, with only extensive coagulative necrosis on resection. Three years after the diagnosis, the patient remains in complete remission.
NHL are a heterogeneous group of lymphoproliferative disorders with distinct clinical and pathologic features. In Brazil, they are among the twelve most frequent cancers in both men and women, with an estimated incidence of 9918 new cases in 2015.4,5
Diffuse large B-cell lymphoma is the most common NHL, accounting for around 30% of adult cases in western countries. Although a percentage of NHL patients may initially have disease confined to extranodal structures, the typical presentation is of a rapidly enlarging tumor mass at a single or multiple nodal sites.1
IBC is a relatively rare clinicopathologic entity that accounts for 2% of invasive breast cancers.2 IBC represents the most aggressive type of breast cancer, being characterized by rapid onset of erythema and edema (peau d’orange) occupying at least one-third of the breast. The skin changes are caused by dermal lymphatic involvement by malignant cells, whose presence on punch biopsy is pathognomonic of IBC. Despite the occasional absence of a palpable breast mass, most cases of IBC have locoregional disease (metastases to axillary/supraclavicular nodes) and about 30% have stage IV metastatic disease at diagnosis. The diagnosis is based on clinical characteristics with essential pathological confirmation of invasive carcinoma.3
The clinical characteristics of IBC are rather unusual for NHLs, being occasionally reported in uncommon cases of primary or secondary breast involvement by lymphomas.6,7 Nodal axillary NHL, however, may also take on the appearance of IBC,8 even in the absence of direct breast involvement as highlighted herein. Presumably, this would occur because of lymph-vascular engorgement and the resultant skin changes.
Although, by routine H&E staining our patient had the initial diagnosis of invasive carcinoma, which makes lymphoma less plausible, NHL may sometimes appear as poorly differentiated carcinoma by conventional histology. Negativity for cytokeratins (e.g. AE1/AE3) and strong positivity for the leukocyte common antigen and CD20 allow the diagnosis of B-cell NHL to be made.9
As the incidence of lymphomas, particularly B-cell lymphomas, is increasing worldwide,1 it is important to be aware of their most uncommon presentations. Axillary nodal NHL with reactive breast/skin changes should then be included among the differential diagnoses of IBC, together with other entities such as acute mastitis, breast lymphoma and extramammary carcinomas metastatic to the breast; such distinction is of paramount importance since the prognoses and management are substantially diverse.