Heparin-induced thrombocytopenia (HIT) is an immune-based side effect of heparin due tothe formation of platelet-activating antibodies, which target platelet factor 4-heparin (PF4/H) complexes and results in thrombocytopenia.1 HIT is typified by a decline in platelet counts that typically occurs within 5 to 14 days after the initiation of heparin therapy and diagnosed based on the evaluation of clinical presentation through a validated pretest probability score, known as the 4Ts score, in combination with PF4/H antibodies corroboration which is a laboratory antigen assay.2 Furthermore, HIT is usually seen patients receiving unfractionated heparin (UFH) and less common with low–molecular weight heparin (LMWH). In this letter, we presented longitudinal changes in the complete blood count (CBC) of confirmed HIT patients in the intensive care units (ICUs).3
This retrospective study was conducted on ICU patients receiving heparin at Rasoul Akram Hospital, Tehran, Iran. Patients with clinical suspicion of HIT were included if they had available PF4 test results and 4Ts score.4 Also, individuals with preexisting heparin allergy or history of coagulopathy, autoimmune disease, and pancytopenia were excluded from the study. Linear mixed model (LMM) was used to assess longitudinal changes in mean CBC components including platelet, hemoglobin, and white blood cell (WBC).
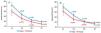
A total of 99 suspected HIT patients were enrolled in the study and divided into positive (n = 35) and negative (n = 64) groups for PF4 antibody (Table 1). A significant difference was observed in the treatment service of patients between positive and negative PF4 groups (p = 0.025), and the total 4T score for positive PF4 group was notably higher than negative PF4 (p = 0.001). Moreover, 88.6 % of positive PF4 patients were high risk for HIT whereas 68.8 % of negative PF4 were intermediate (p = 0.001). Mean platelets showed a significant decrease in positive PF4 patients for both genders (p = 0.001) over the time of heparin therapy (3rd, 5th, 7th day) while changes in mean hemoglobin level and WBC were not significant (Table 2). Figure 1 represents changes in mean platelets for different genders (Figure 1A) and treatment services (Figure 1B) during heparin therapy in positive PF4 patients.
Demographic and clinical data of ICU patients in two groups of study (n = 99).
| Patients’ variables | Positive PF4 (n = 35) | Negative PF4 (n = 64) | P value |
|---|---|---|---|
| Age (year)a | 63.94±17.51 | 64.06±17.80 | 0.974 |
| Gender (n,%)b | |||
| Male | 19 (54.3) | 41 (64.1) | 0.341 |
| Female | 16 (45.7) | 23 (35.9) | |
| Treatment service (n,%)b | |||
| Internal patients | 16 (45.7) | 44 (68.7) | 0.025 |
| Surgical patients | 19 (54.3) | 20 (31.3) | |
| Type of heparin (n,%)b | |||
| UHF | 33 (94.3) | 57 (89.1) | 0.387 |
| LMWH | 2 (5.7) | 7 (10.9) | |
| Indication for heparin (n,%)b | |||
| Treatment | 16 (45.7) | 20 (31.3) | 0.153 |
| Prophylaxis | 19 (54.3) | 44 (68.8) | |
| Comorbidities (n,%)b | |||
| Hypertension | 21 (60) | 27 (42.2) | 0.090 |
| Valvular heart disease | 1 (2.9) | 1 (1.6) | 0.662 |
| Atrial fibrillation | 4 (11.4) | 5 (24) | 0.550 |
| Ischemic heart disease | 14 (40) | 29 (45.3) | 0.610 |
| Hear failure | 4 (11.4) | 9 (14.1) | 0.711 |
| Diabetes | 6 (17.1) | 21 (32.8) | 0.094 |
| Stroke | 5 (14.3) | 9 (14.1) | 0.976 |
| Chronic kidney disease | 4 (11.4) | 17 (26.6) | 0.078 |
| COPD | 5 (14.3) | 7 (10.9) | 0.626 |
| Deep vein thrombosis | 3 (8.6) | 6 (9.4) | 0.894 |
| Pulmonary embolism | 6 (17.1) | 3 (4.7) | 0.039 |
| Cancer | 5 (14.3) | 10 (15.6) | 0.859 |
| APACHE II score a | 7.02±15.43 | 4.47±11.85 | 0.361 |
| SOFA score a | 8.74±1.26 | 9.03±1.39 | 0.312 |
| Total 4T score | 6.31 ± 0.71 | 4.67±1.00 | 0.001 |
| Low risk (n,%) | 0 | 8 (12.5) | |
| Intermediate risk (n,%) | 4 (11.4) | 44 (68.8) | 0.001 |
| High risk (n,%) | 31 (88.6) | 12 (18.7) |
Abbreviations: ICU, intensive care unit; PF4, platelet factor 4; UHF, unfractionated heparin; LMWH, low molecular weight heparin; COPD, chronic obstructive pulmonary disease; APACHE, Acute Physiology and Chronic Health Evaluation; Sequential Organ Failure Assessment.
Complete blood count components of positive PF4 patients during heparin therapy in ICUs.
| Variables* | Gender | Duration of heparin therapy | p value | |||
|---|---|---|---|---|---|---|
| 1 day | 3 day | 5 day | 7 day | |||
| Platelets | Male | 241.89±91.10 | 173.47±84.46 | 122.42±69.94 | 103.0 ± 45.86 | 0.001 |
| Female | 206.44±105.60 | 141.0 ± 69.69 | 123.25±56.67 | 113.46±59.18 | 0.001 | |
| Hemoglobin | Male | 11.63±3.54 | 10.30±2.74 | 11.13±2.30 | 10.62±3.17 | 0.541 |
| Female | 11.10±2.29 | 10.86±1.39 | 9.88±1.19 | 10.06±1.98 | 0.156 | |
| WBC | Male | 11.46±5.08 | 10.62±3.61 | 9.21±3.61 | 8.76±3.65 | 0.147 |
| Female | 10.71±3.39 | 11.66±3.49 | 11.31±3.85 | 10.80±4.34 | 0.650 | |
Abbreviations: PF4, platelet factor 4; ICU, intensive care unit; WBC, weight blood cell.
Age, gender, treatment service, and duration of heparin therapy were considered as covariates in LMM analysis to find their effects on longitudinal changes in CBC components. Our results showed that changes in mean platelets in surgical patients were remarkably greater than in internal patients (β= −32.6, p = 0.03). Moreover, duration of the patient's exposure to heparin significantly affected longitudinal changes in mean platelets in positive PF4 patients ([3rd day: β= −6.13; p = 0.001], [5th day: β= −97.1; p = 0.001], [7th day: β= −112.2; p = 0.001]). A significant relationship was found between changes in mean hemoglobin and gender (β= −1.7, p = 0.005) and treatment service (β= −1.3, p = 0.04) of positive PF4 patients over the time of heparin therapy. Age of patients did not affect longitudinal changes in CBC components.
The occurrence rate of HIT in patients on heparin is highly variable. HIT is commonly less frequent in patients receiving low molecular weight heparin (LMWH) (< 1 %) in comparison with UHF (1–5 %).5 Moreover, females are more prone to develop HIT than males. Consistent with us, the rate of HIT occurrence in surgical ICU patients has been shown to be higher than in medical ICU patients.6 In surgical patients treated with UFH, the risk of HIT is 10 to 15 times higher than in those treated with LMWH. Heparin administration prophylactically runs the risk of antibody formation, while clinical presentations observe more in patients treating with therapeutic doses. Just 5 % to 30 % of cases with the production of HIT IgG antibodies will develop HIT.7 The main causes of thrombocytopenia in HIT patients are major surgical procedures, transfusion reactions, side effects of drugs, and sepsis. It has been recommended that monitoring of thrombotic complications in patients on heparin can be a better indicator of HIT diagnosis than uncomplicated thrombocytopenia.8 In uncomplicated patients with low susceptibility to HIT, administration of heparin should not be stopped and additional laboratory testing is not necessary. But heparin administration should be discontinued and alternative anticoagulation should be initiated in patients with an increased susceptibility to HIT.9
Based on the findings of this study, duration of the patient's exposure to heparin affects significantly magnitude of decline in platelets of patients with PF4/H antibody and may result in discontinuing heparin administration. Also, the degree of platelet decline in surgical HIT patients is notably greater than in internal HIT patients.
This study has been approved by the local Ethics Committee (IR.IAU.KHUISF.REC.1397.152), and written informed consent was obtained from all individuals.