Sickle cell anemia (SCA) is a hereditary hemoglobinopathy caused by homozygosity for sickle hemoglobin (Hb S). Hb S is caused by a single mutation in the β-globin gene (HBB, glu6val) resulting in the presence of valine instead of glutamic acid in the sixth position of the β-globin chain.1,2 Hb S can form polymers when deoxygenated leading to vaso-occlusion and tissue ischemia which may result in severe pain of the chest, back, abdomen, or extremities.2,3
Acute chest syndrome (ACS) is an acute illness characterized by fever and/or respiratory symptoms, accompanied by new pulmonary infiltrates on a chest X-ray. 4–10 It is the leading cause of hospitalization and intensive care unit admission,4,6 as well as the most common cause of death in adult patients with SCA even among those on hydroxyurea (HU) therapy.8,9 Factors such as infection, fat embolism, iatrogenic fluid overload, hypoventilation from pain or narcotic analgesics, and vaso-occlusion of the pulmonary vasculature contribute to the pathogenesis of ACS.1,2,7 Neurologic complications following ACS occur in about 22% of adults and 8% of children with SCA.10 The pathophysiology of neurological deficits in patients with ACS seems to be related to an abrupt decrease in oxygenation in the vascular bed of the central nervous system.
Acute ischemic stroke (AIS) is a devastating neurological complication of SCA, occurring in about 8% of Hb SS children by their second decade of life. Children with SCA and strokes frequently present with vasculopathy in the distal internal carotid and middle cerebral arteries, although extracranial vasculopathy can also be present.2 Other less common neurologic complications of SCA include hemorrhagic stroke, transient ischemic attack, silent cerebral infarct, dural venous sinus thrombosis, posterior reversible encephalopathy syndrome (PRES), and migraine.5,11,12
Although occlusion of cervical carotid arteries is a known cause of AIS, these vessels are not routinely screened in sickle cell disease. These patients are commonly examined with neurological imaging such as magnetic resonance imaging (MRI); magnetic resonance angiography and transcranial Doppler sonography (TCD). However, these exams are mainly carried out to identify lesions in the major intracerebral vessels.12
We report a case of an adult SCA patient who presented with ACS followed by cardiac arrest and coma due to cerebral hypoperfusion.
Case reportA 23-year-old female with SCA and a past medical history of two episodes of ischemic stroke was admitted to hospital due to fever, tachypnea, thoracic pain, hypoxemia, cough and wheezing. She had been on chronic blood transfusions for 14 years since her first stroke and had also been taking hydroxyurea for two years. This strategy was chosen due to the difficulty in maintaining the ideal interval between transfusions as a result of alloimmunization.
Auscultation of the chest revealed reduced breath sounds at the left pulmonary base. A chest X-ray showed a left lower lobe infiltrate and the oxygen saturation was 95% with a continuous positive airway pressure using 50% oxygen. Despite treatment for ACS (supportive care, fluid management, oxygenation, chest physiotherapy and antibiotics), two days after hospital admission the patient suffered cardio-respiratory arrest. After a successful resuscitation, although she responded to pain, she had no spontaneous eye opening and no verbal response. She also had bilateral deviation of eye movement to the right, but no other clinical signs of meningeal irritation. The diagnosis of coma (Glasgow Coma Score 5) was subsequently made.
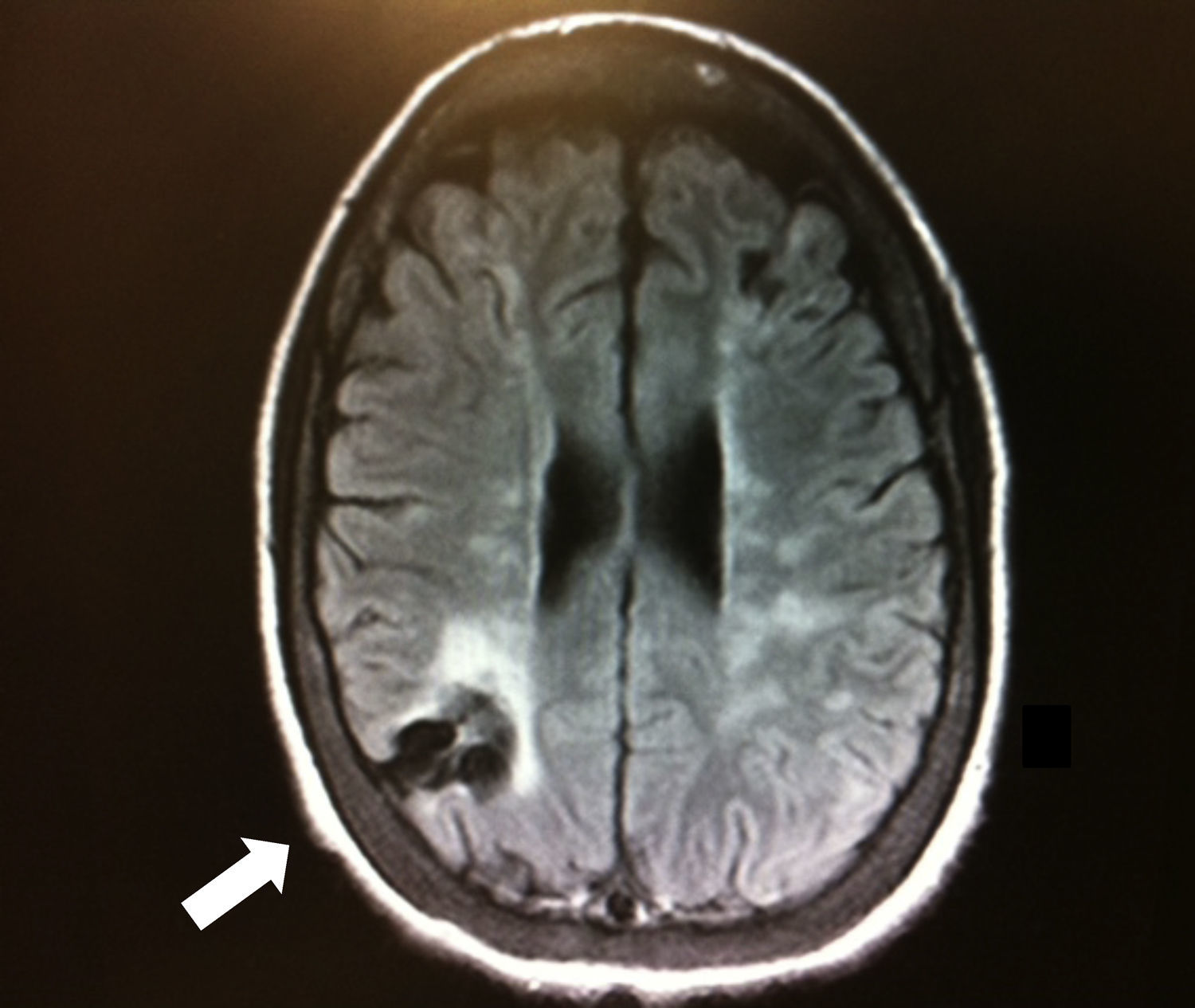
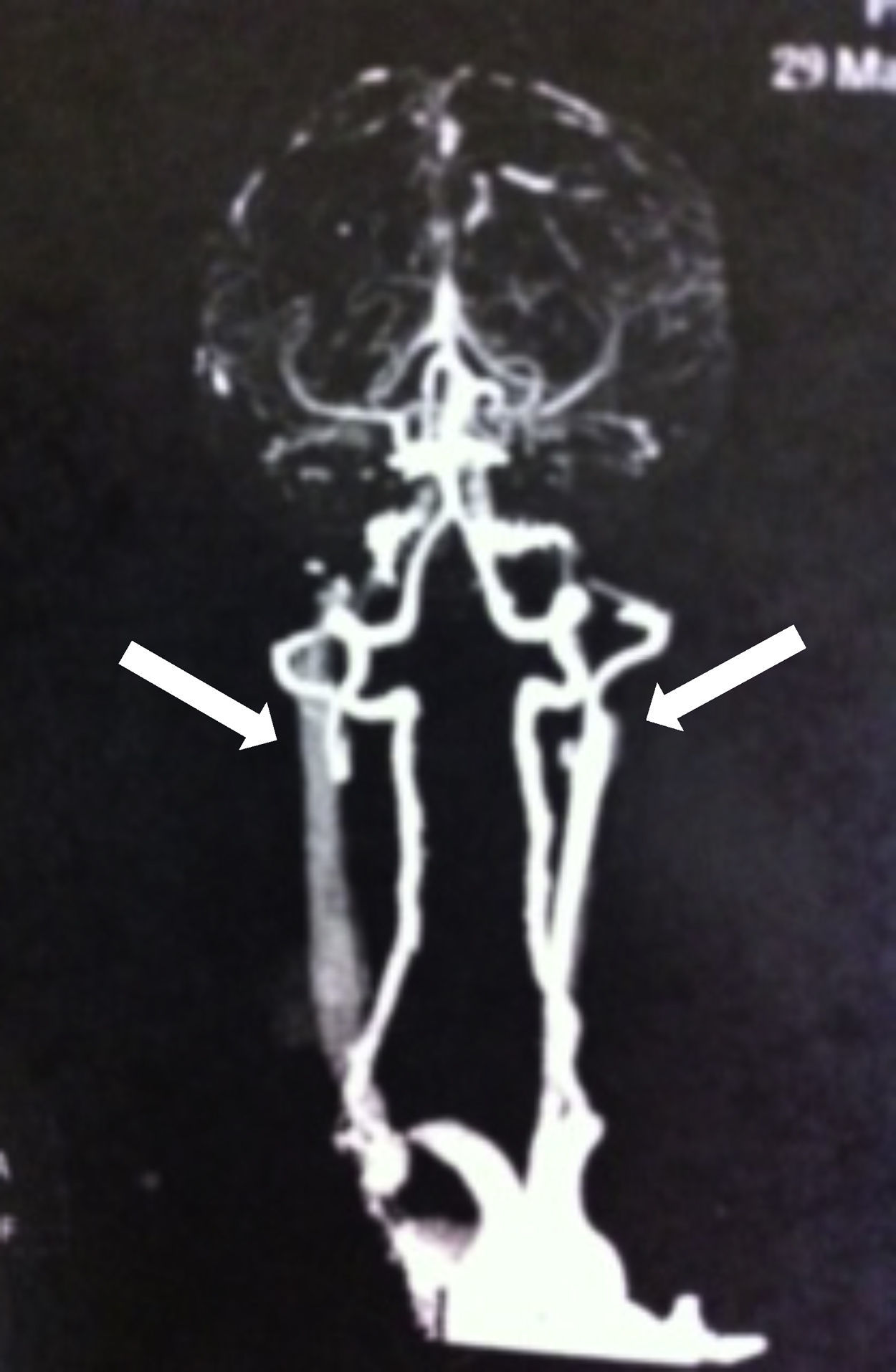
Brain magnetic resonance imaging after the cardiac arrest showed only old ischemic areas compatible with her previous strokes (Figure 1). A computerized tomography angiography revealed severe stenosis of the left cervical internal carotid artery and occlusion of the right cervical internal carotid artery (Figure 2). TCD showed symmetric but decreased blood flow velocities in both middle cerebral arteries (time-averaged maximum mean velocity: 40cm/s), as well as collateral flow from the posterior circulation.
She remained in coma for 19 days and during this period she was treated with multiple exchange blood transfusions in order to maintain the Hb S concentration at <30%. She had full neurological recovery without new deficits (Glasgow Coma Score 15). She has been followed up and treated with chronic blood transfusions and acetylsalicylate (aspirin) for the last three years.
DiscussionWe describe a patient with SCA who presented with ACS complicated by cardiac arrest and coma. Despite having bilateral cervical carotid artery disease, the patient had full neurological recovery. In patients resuscitated from cardiac arrest, the neurological outcome depends on immediate restoration of systemic circulation and oxygenation to meet the cerebral oxygen demand.13 In our patient, cerebral flow was probably even more compromised during cardiac arrest because of bilateral cervical carotid artery disease.
Although there are several reports of ischemic stroke associated with lesions in the cervical internal carotid artery detected at autopsy by digital subtraction angiography and by magnetic resonance angiography, most patients with SCA and stroke are not screened for cervical artery disease, and neuroimaging is usually restricted to the major intracerebral vessels.14–17 Our patient had had two previous strokes, however we did not have an evaluation of her intracranial vasculature before the cardiac arrest.
Collateral circulation can develop in patients with intracranial and cervical artery occlusions. For instance, major collateral anastomoses can occur between the ophthalmic branch of the internal carotid artery and the terminal twigs of the internal and external maxillary tributaries of the external carotid arteries. Adequate collateral vessels confer protection in the event of ischemic lesions.16 Our patient had low cerebral blood flow velocities in the anterior circulation with robust collateral flow through the posterior circulation. Her excellent neurological outcome was probably related to the presence of posterior circulation collateral vessels.
The management of symptomatic cervical carotid artery disease in patients without SCA is well established, with revascularization being the gold standard treatment.18–20 In patients with SCA and cervical carotid artery disease, the impact of therapies such as endarterectomy or angioplasty with stenting is uncertain. Rather, patients continue to be managed with chronic blood transfusions similar to those with intracranial carotid artery disease.
In conclusion, a good neurological outcome in patients with bilateral cervical carotid artery disease is possible even in the setting of extremely low cerebral blood flow as seen during cardiac arrest. The role of collateral circulation between extra- and intra-cranial vessels in patients with SCA needs further evaluation since this could influence the decision to consider revascularization therapies in patients with cervical artery disease. Also, the value of cervical carotid artery Doppler ultrasound scanning should be studied prospectively, since this quick and non-invasive procedure may identify patients at increased risk of stroke who would otherwise be misdiagnosed as PRES or several other differential diagnosis of SCA patients with acute neurologic manifestations.
Conflicts of interestThe authors declare no conflicts of interest.
A.C.P.M. was supported by FAPESP (12/19346-1)