
Additional cytogenetic abnormalities (ACA) are known to crop up in Ph+ cells of chronic myeloid leukemia (CML) patients due to cytogenetic evolution. But the frequency of molecular evolution and ACA is much less in Ph− cells of CML patients and is poorly understood. We report an interesting and rare case of Ph+ CML, who progressed to B lymphoblastic crisis, achieved remission, and later developed Ph− acute myeloid leukemia (AML) with KMT2A gene rearrangement and no detectable BCR- ABL transcripts.
Chronic myeloid leukemia (CML) is characterized by the chromosomal translocation t(9;22)(q34.1;q11.2) resulting in the formation of the Philadelphia (Ph) chromosome.1 Significant improvement in prognosis and survival rate was seen after the introduction of tyrosine kinase inhibitors (TKI) in the treatment protocol. But over the years of treatment, additional cytogenetic abnormalities (ACA) are known to crop up in Ph+ cells due to cytogenetic evolution.2 The most common ACA include trisomy 8 and trisomy 19 and extra copy of Ph chromosome and i(17) (q10). The evolution of ACA in Ph+ cells predicts a poorer response to TKIs and a higher risk of progression.3
But the frequency of molecular evolution and ACA is much less in Ph− cells of CML patients and is poorly understood.4 The most frequent ACAs in Ph− cells in CML patients were trisomy 8 and monosomy 7. We report an interesting and rare case of Ph+ CML, who progressed to B lymphoblastic crisis, achieved remission, and later developed Ph− acute myeloid leukemia (AML) with KMT2A gene rearrangement and no detectable BCR::ABL transcripts.
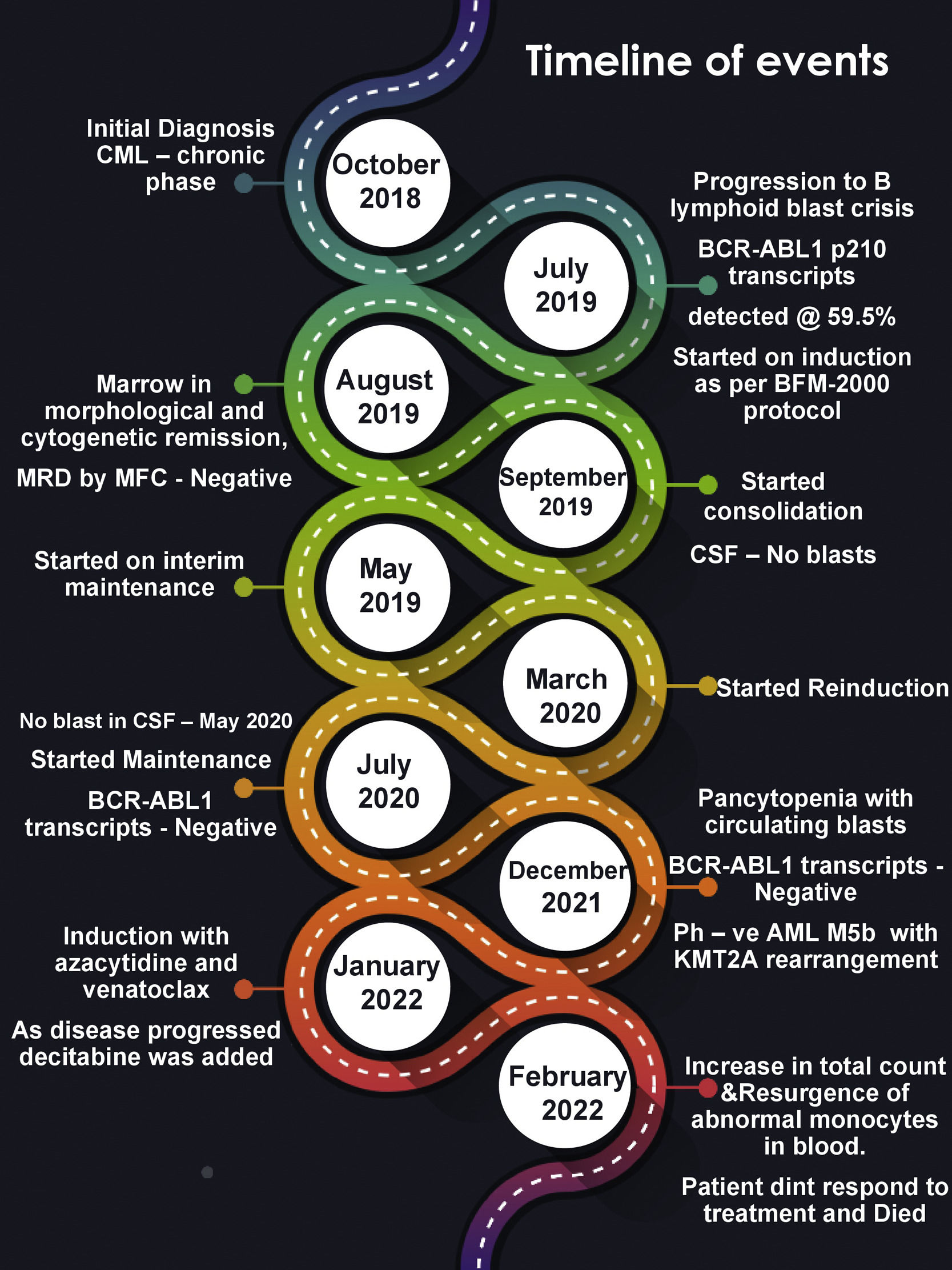
Case presentationA 44-year-old gentleman presented to our outpatient department on July 2019 with complaints of low-grade fever, night sweats, and lack of sleep. He was diagnosed earlier as a case of CML - Chronic phase in October 2018 and was on tablet imatinib 400 mg once daily since then. The past records of periodic BCR::ABL1 monitoring were not available. Physical examination was unremarkable except for mild hepato-splenomegaly. The CT scan revealed hepato-splenomegaly, multiple (celiac, perigastric, para-aortic, iliac, and mesenteric) lymphadenopathy, and lepto meningeal deposits.
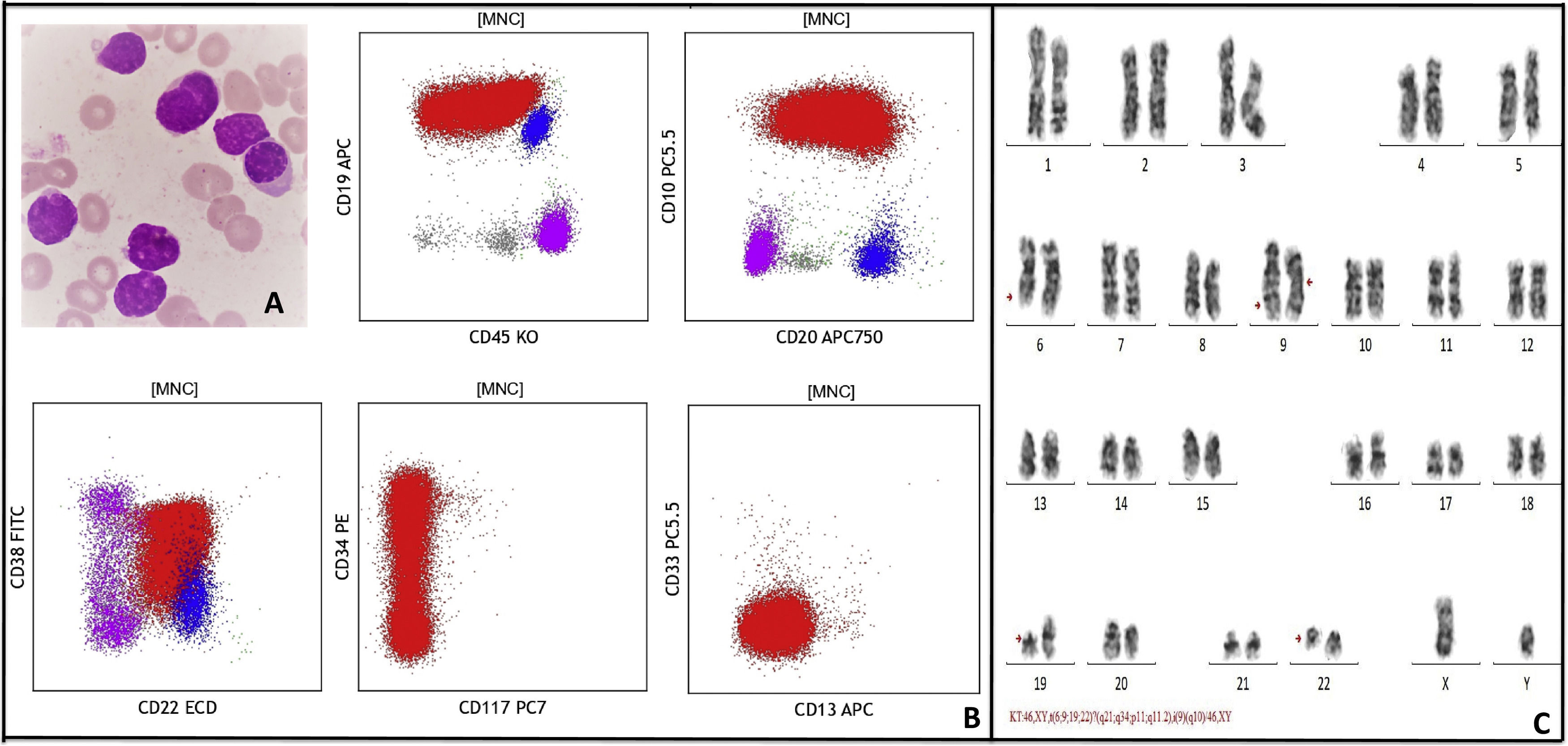
His complete blood counts showed hemoglobin (Hb) of 9.0 g/dl, total leucocyte count (TLC) of 19.9 × 106/L, and platelet count (PLT) of 60 × 106/L. Peripheral blood smear showed 75% blasts population (Fig. 1a). Peripheral blood immunophenotyping (IPT) showed 77.6% B lymphoid blasts expressing CD10 (bright), CD19 (bright), CD20 (variable), CD22 (dim-moderate), CD79a (moderate), IgM (dim), CD34 (subset) and negative for T cell and myeloid markers (Fig. 1b). Fluorescence in situ hybridization (FISH) detected BCR::ABL1 [Abott vysis BCR/ABL1/ASSN1 tri-color dual fusion probe) in 69% of interphase nuclei. Quantitative real time polymerase chain reaction (RT-PCR) in peripheral blood sample detected BCR::ABL1 p210 transcripts at 59.5%. Conventional cytogenetic evaluation of the leukemic blood revealed complex abnormal karyotype with four-way translocation involving long arm (q21) of chromosomes 6 and short arm (p11) of chromosome 19 in addition to translocation between long arm (q34 and q11.2) of chromosomes 9 and 22. The other chromosome 9 was an isochromosome showing two copies of long arm [i(9q)] (Fig. 1c).
A: Peripheral blood smear at blast crisis showing many lymphoid blasts, B: Flow cytometry dot plots show B lymphoid blasts (red) expressing CD19 (bright), CD10 (bright), CD34 (variable), CD20 (variable), CD45 (variable), and negative for CD117, CD13, and CD33. Normal B cells (blue) and T cells (pink) are also seen, C: Representative karyotype of a metaphase cell showing 46,XY,t(6;9;19;22)?(q21;q34;p11,q11.2),i(9)(q10)[14]/46,XY[2].
A diagnosis of Ph+ B lymphoblastic crisis in a known case of CML was made. He was started on Berlin-Frankfurt-Munich (BFM) - 2000 protocol with tablet dasatinib 140 mg once daily. He was tolerating the treatment well. The end of induction marrow showed morphological, cytogenetic remission. Measurable residual disease was also undetected by multiparametric flow cytometry (MFC). The patient was continued with consolidation treatment as per the protocol with dasatinib. The cerebro spinal fluid (CSF) cytology examination for blasts was done during the intrathecal methotrexate administration at different time points and were all negative. The patient did not have a matched sibling donor and was not willing for an allogeneic bone marrow transplantation. Hence the patient was further continued on interim maintenance (protocol M) followed by re-induction chemotherapy and maintenance phase. After a regular follow-up for one year, (in August 2020) the patient achieved deep molecular response (BCR::ABL1 p210 transcripts <0.01) during his course on maintenance treatment.
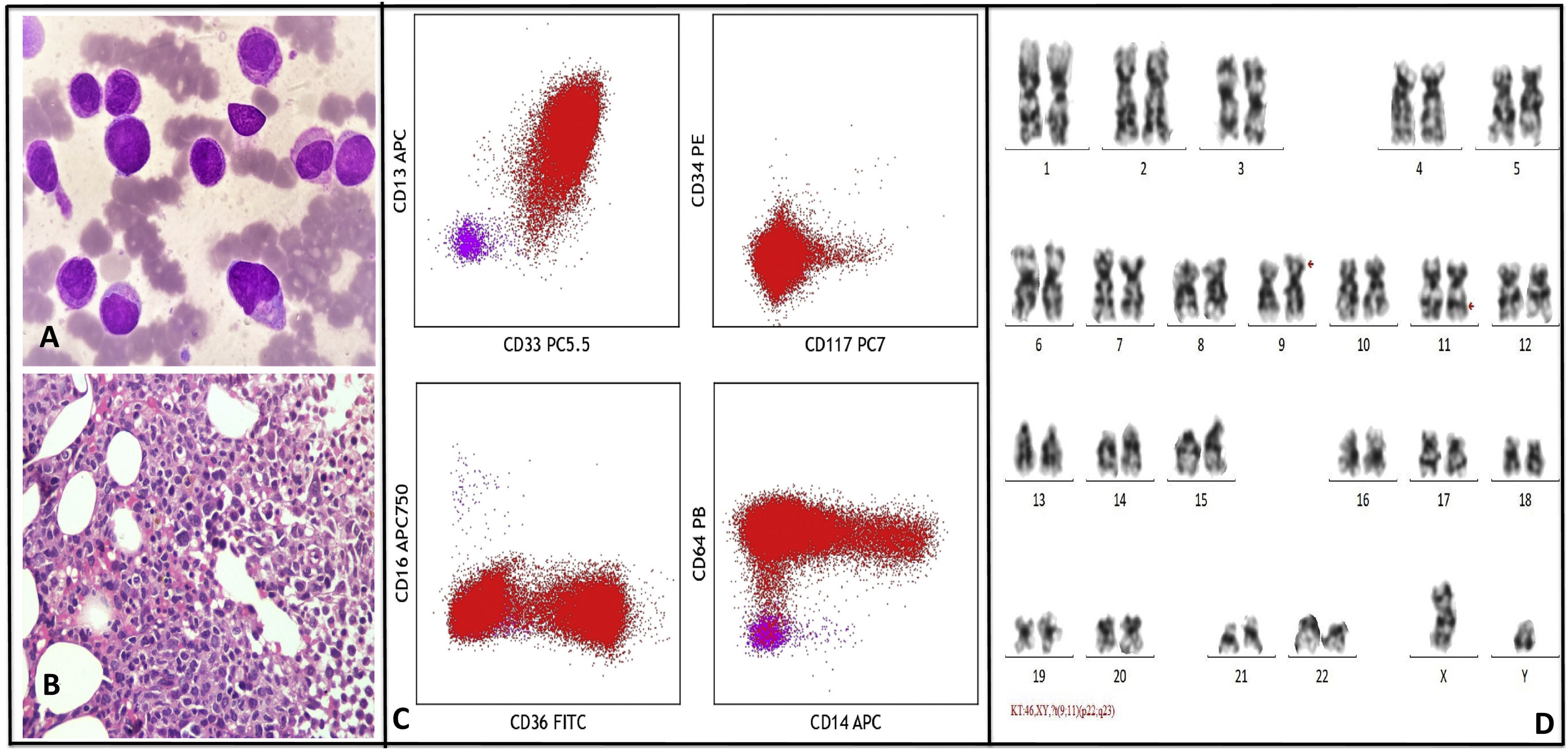
In January 2022, the patient presented with complaints of mild grade fever and recent weight loss. The CBC showed pancytopenia (Hb - 9 mg/dl, TLC - 0.7 × 106/L, PLT - 25 × 106/L). Bone marrow aspirate cytology (BMA) was consistent with AML with monocytic differentiation (Fig. 2a). BMA IPT revealed abnormal promonocytes/ monocytes population with high side scatter quantified at 72.9% expressing CD33 (moderate-bright), CD13 (moderate), CD64 (moderate), CD4(dim), CD36 (subset), CD15(variable), CD14 (subset), CD11b (subset), cyto MPO (moderate) and negative for CD34 & CD117 (Fig. 2c).
A: Bone marrow aspirate show many promonocytes and abnormal monocytes, B: Marrow trephine biopsy show sheets of promonocytes and monocytes, C: Flow cytometry dot plots show abnormal monocytic population expressing CD33 (moderate), CD13 (variable), CD64 (moderate), CD36 (subset), CD14 (variable) and negative for CD34 and CD117. Normal T cells (pink) are also seen, D: Representative karyotype of a metaphase cell showing 46,XY,?t(9;11)(p22;q23)[20].
A final diagnosis of AML M5b was made by morphology and IPT findings. Bone marrow trephine biopsy was hypercellular and showed sheets of promonocytes and monocytes which were positive for CD68, CD4 and MP by immunohistochemistry (IHC), consistent with AML (Fig. 2b). Surprisingly FISH was positive for KMT2A gene rearrangement (Abott vysis LSI MLL dual color, break-apart rearrangement probe) and was negative for BCR-ABL fusion (tri-color dual fusion probe). Quantitative RT-PCT was negative for BCR::ABL1 p210, p190 and p230 transcripts. Karyotyping done on BMA sample showed a new abnormal karyotype t(9;11)(p22;q23) in all the 20 metaphases analyzed (Fig. 2d). Next generation sequencing with oncomine myeloid panel (Thermo–Fischer ion torrent PGM) showed KMT2A-MLLT3 fusion t(9;11)(p22;q23). No other mutations were identified.
He was started on AML induction treatment with azacitidine and venetoclax. On day 6, the abnormal monocytes were cleared from the peripheral blood but only to reappear after a few days. Post which he was treated with an increased dose of venetoclax along with decitabine, cyclophosphamide, cytarabine, and topotecan. But he did not respond to the treatment and died.
DiscussionWe have reported a unique case of CML in Chronic Phase, that progressed to B- lymphoid blast crisis. After undergoing treatment for 2.5 years, he developed AML with KMT2A rearrangement and no detectable BCR::ABL transcripts (Fig. 3). KMT2A rearrangement is commonly reported in cases of infantile acute leukemia and therapy related leukemias in adults. The occurrence of 11q23/KMT2A rearranged (Ph–) AML in a case of CML is extremely rare.
Two possible mechanisms have been postulated for the development of Ph− AML in CML cases. The first hypothesis states that the Ph− stem cells have a genetic abnormality that induces clonal hematopoiesis with many chromosomal abnormalities including the t(9;22). After the TKI therapy, the Ph+ clones get suppressed and the Ph− clone becomes evident. Further, the presence of abnormalities in chromosome 7 and 11 in Ph− and Ph+ clones is consistent with clonal hematopoiesis before the emergence of the Ph chromosome.5 Our case did not show abnormalities of chromosome 7 and 11 at diagnosis or blast crisis. The second possibility is both CML and AML arise independently and are clonally unrelated. Our case did not show any cytogenetic abnormalities found at diagnosis or lymphoid blast crisis, in the later developed Ph− MLL rearranged AML clone. This may suggest that Ph− AML developed independently and clonally unrelated to CML. Another hypothesis is related to TKI therapy, as imatinib suppresses the activity of cytoplasmic ABL, which is important for the DNA repair pathway.6 A significant study done by Jabbour et al. in a cohort of 258 CML patients on imatinib therapy showed that 9% of patients developed chromosomal abnormalities in Ph− cells.7
This report may influence us to a conclusion of monitoring of all CML patients on TKI by conventional cytogenetics even after achieving the desired molecular response. Moreover, the incidence of cytogenetic abnormalities in Ph− cells of patients who achieved complete cytogenetic response ranges from 10 to 20%. But given its potential clinical implication, large studies with systematic data collection is necessary for a decisive opinion. Registries collecting data on patients who develop chromosomal abnormalities in ph- cells are coming up. This may help in giving clarity on the need for cytogenetic monitoring in CML patients on TKI therapy.8–10
Chemotherapy is very crucial in the treatment of CML cases with blast crisis or Ph− acute leukemia. The use of TKI therapy in CML cases with Ph− acute leukemia is unclear. If the patient shows complete remission for BCR::ABL1, then TKI can be withheld with continuation of chemotherapy. Stem cell transplantation is another option which may give durable remission. However, the prognosis of Ph− AML arising in a CML case is dismal.5
ConclusionAcute leukemia arising in a known case of CML should not be presumed as a CML blast crisis unless BCR::ABL1 transcripts are detected by molecular genetic studies. CML patients on regular TKI therapy should be closely monitored for cytogenetic abnormalities in the Ph− cells.10 Molecular techniques like next generation sequencing can help in predicting the risk of developing secondary hematological malignancies in CML cases during and after TKI therapy.11
Nil.


![A: Peripheral blood smear at blast crisis showing many lymphoid blasts, B: Flow cytometry dot plots show B lymphoid blasts (red) expressing CD19 (bright), CD10 (bright), CD34 (variable), CD20 (variable), CD45 (variable), and negative for CD117, CD13, and CD33. Normal B cells (blue) and T cells (pink) are also seen, C: Representative karyotype of a metaphase cell showing 46,XY,t(6;9;19;22)?(q21;q34;p11,q11.2),i(9)(q10)[14]/46,XY[2]. A: Peripheral blood smear at blast crisis showing many lymphoid blasts, B: Flow cytometry dot plots show B lymphoid blasts (red) expressing CD19 (bright), CD10 (bright), CD34 (variable), CD20 (variable), CD45 (variable), and negative for CD117, CD13, and CD33. Normal B cells (blue) and T cells (pink) are also seen, C: Representative karyotype of a metaphase cell showing 46,XY,t(6;9;19;22)?(q21;q34;p11,q11.2),i(9)(q10)[14]/46,XY[2].](https://static.elsevier.es/multimedia/25311379/00000046000000S6/v2_202501280810/S253113792300041X/v2_202501280810/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w93OM6WmS6o9DeZl+SVh74uo=)
![A: Bone marrow aspirate show many promonocytes and abnormal monocytes, B: Marrow trephine biopsy show sheets of promonocytes and monocytes, C: Flow cytometry dot plots show abnormal monocytic population expressing CD33 (moderate), CD13 (variable), CD64 (moderate), CD36 (subset), CD14 (variable) and negative for CD34 and CD117. Normal T cells (pink) are also seen, D: Representative karyotype of a metaphase cell showing 46,XY,?t(9;11)(p22;q23)[20]. A: Bone marrow aspirate show many promonocytes and abnormal monocytes, B: Marrow trephine biopsy show sheets of promonocytes and monocytes, C: Flow cytometry dot plots show abnormal monocytic population expressing CD33 (moderate), CD13 (variable), CD64 (moderate), CD36 (subset), CD14 (variable) and negative for CD34 and CD117. Normal T cells (pink) are also seen, D: Representative karyotype of a metaphase cell showing 46,XY,?t(9;11)(p22;q23)[20].](https://static.elsevier.es/multimedia/25311379/00000046000000S6/v2_202501280810/S253113792300041X/v2_202501280810/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w93OM6WmS6o9DeZl+SVh74uo=)





