The clinical phenotype of sickle cell/beta+-thalassemia (Hb S/β+-Thal) is highly variable, and severity is associated with the quantitative degree of decrease in the production of the beta globin chains.1 Evidence shows that differences in the production of hemoglobin A (Hb A) and severity correspond to different molecular beta-thalassemia (β-Thal) mutations.2 A previous report proposed a classification of Hb S/β+-Thal phenotypes based on the relative concentration of Hb A: Type I: 1–7% of Hb A; Type II: 7–14% of Hb A; and Type III: 14–25% of Hb A.2 However, some β-Thal mutations lead to low impairment of β-globin production and the resulting phenotype does not fit this proposed classification. In a single patient case report, the −92 (C>T) mutation was associated with a high level of Hb A (45%) in an adult Sicilian patient with Hb S/β+-Thal.3 Recently, a combination of two sequence variants, IVS-II-839 (T>C) and IVS-II-844 (C>A), was associated with a very mild phenotype of sickle cell disease (SCD).4 Because of the marked clinical variability of Hb S/β+-Thal patients, molecular predictors of disease severity would be helpful to guide treatment choices in children with this genotype. The objective of this study was to characterize the hematological parameters, clinical features, and molecular basis of very mild forms of Hb S/β+-Thal in a newborn cohort of Minas Gerais state, Brazil.
Case seriesThis is a case series involving children from the Minas Gerais State SCD newborn cohort, Brazil. As part of a yet incomplete study, 56 out of 96 (58.3%) children with Hb S/β0-thal or Hb S/β+-Thal from that cohort born between 1998 and 2013 had already been submitted to DNA analysis to identify the β-thal mutations causing Hb S/β-thal. Children were considered to be eligible for this report if they had Hb A concentrations equal to or above 25% confirmed by hemoglobin electrophoresis. Children had hemoglobin FSA patterns diagnosed by isoelectric focusing and high-performance liquid chromatography in the Newborn Screening Program and have been followed up since diagnosis in the outpatient care unit of Fundação Hemominas, which is situated in the state capital, Belo Horizonte.
Laboratory and clinical data were retrieved by chart review after approval of the local institutional review board. Hematologic and genetic studies were performed on the parents of two children to elucidate the inheritance pattern of the β-thal mutations.
Complete blood count was performed using an electronic cell counter (model T-890, Beckman Coulter, Hialeah, FL, USA or Cell-Dyn ruby, Abbott, IL, USA). Hemoglobin electrophoresis was conducted in an alkaline medium (SPIFE kits, Helena Laboratories, Beaumont, TX, USA) and the percentage of Hb F was quantified by radial immunodiffusion (HbF QUIPlate, Helena Laboratories, Beaumont, TX, USA). The reticulocyte count was measured by optical microscopy using brilliant cresyl blue.
Genomic DNA was isolated with a QIAGEN kit (QIAamp® DNA Blood Mini Kit, Qiagen, Hilden, Germany). The HBB gene was amplified with specific primers. Sequence data were generated with an ABI 3130xl capillary sequencer (Applied Biosystems, Foster City, CA, USA) using standard protocols. A multiplex gap polymerase chain reaction (PCR) assay was used to detect the most common alpha-thalassemia deletions.5
Written informed consent was obtained from parents or guardians of every child in accordance with the norms of the Declaration of Helsinki guidelines with the child's assent being obtained when appropriate.
The study included four unrelated children with diagnosis of Hb S/β+-Thal. The mean age was 8.0±2.4 years (range: 5.5–11.2 years) and all were male. These children were followed for a mean of 7.7±2.4 years (range: 5.2–11 years).
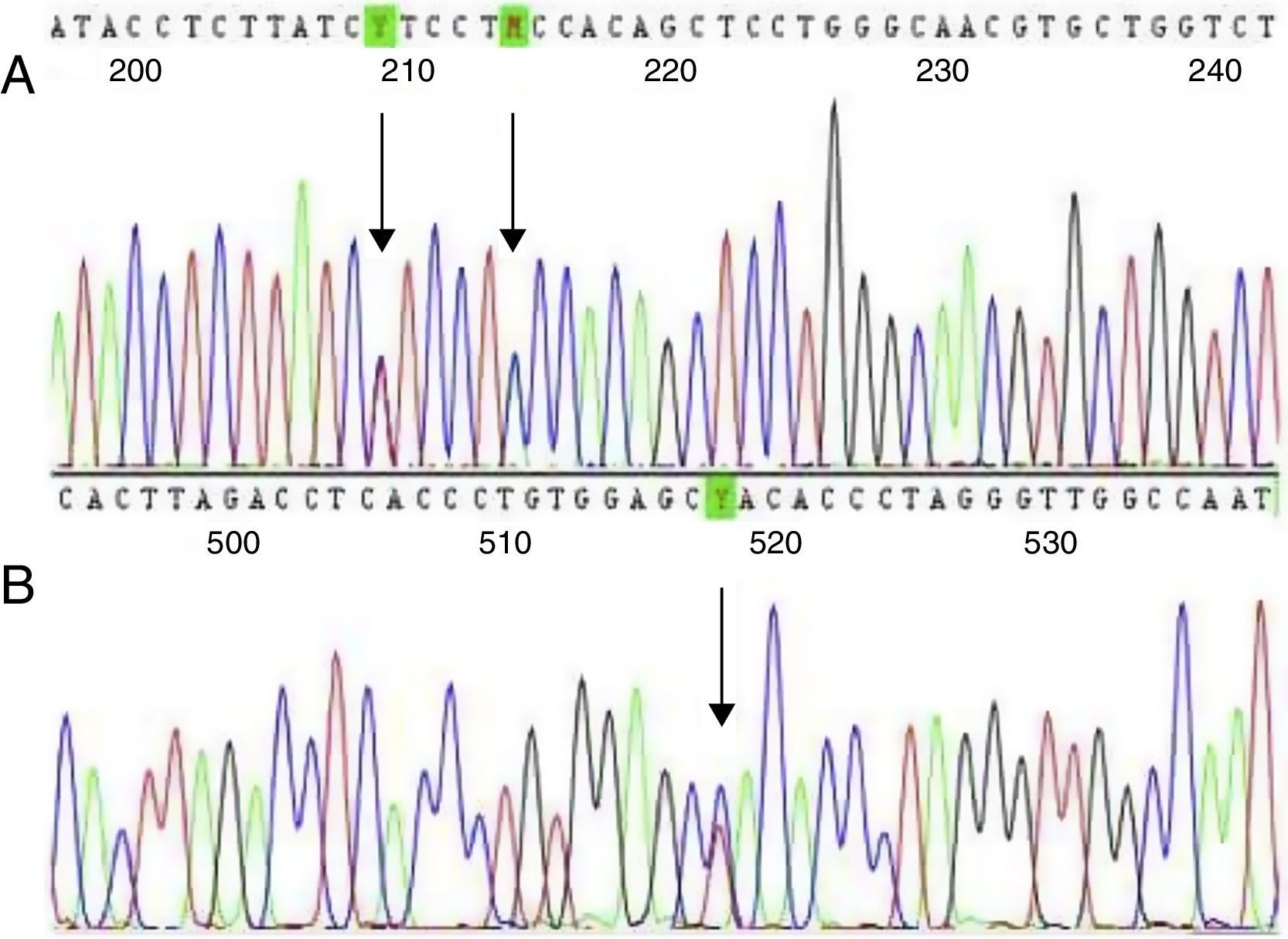
Two children had a −92 (C>T) mutation (HBB:c.−142C>T) and two had IVS-II-844 (C>A) (HBB:c.316-7C>A) plus IVS-II-839 (T>C) (HBB:c.316-12T>C) mutations (Figure 1). Genetic family studies showed that the IVS-II-844 (C>A) and IVS-II-839 (T>C) mutations were in cis in both children because both mutations were inherited from just one of the parents. No child had co-inherited alpha-thalassemia deletions. The mean relative concentration of Hb A was 40.4% and that of Hb S was 54%. Genetic, laboratory, and family data are summarized in Table 1.
Genetic, laboratory, and family characteristics of children with very mild forms of Sβ+-thal in Minas Gerais, Brazil.
| Family I | Family II | Family III | Family IV | |||||
|---|---|---|---|---|---|---|---|---|
| Family relationship | Child I | Mother | Father | Child II | Mother | Father | Child III | Child IV |
| Beta-globin genotype | βS/βThalIVSII−844/839 | βA/βThalIVSII−844/839 | NA | βS/βThalIVSII−844/839 | βS/βA | βA/βThalIVSII−844/839 | βS/βThal−92C>T | βS/βThal−92C>T |
| Age (years) | 7.4 | 8.0 | 5.5 | 11.2 | ||||
| Gender | Male | Female | Male | Male | Female | Male | Male | Male |
| Hemoglobin (g/dL) | 12.9±0.4 | 11.2 | 16.1 | 12.0±0.8 | 14.5 | 16.8 | 11.4±1.1 | 11.5±0.6 |
| Hematocrit (%) | 40±1.1 | 35.1 | 47.1 | 37.5±2.5 | 43 | 50.2 | 35.4±2.1 | 35.3±1.4 |
| Red blood cells (106/μL) | 5.5±0.3 | 4.1 | 5.5 | 5.3±0.3 | 4.5 | 5.8 | 4.7±1.1 | 4.6±0.2 |
| Mean corpuscular volume (fL) | 74.5±3.4 | 85.7 | 85.3 | 75.0±1.1 | 95.5 | 86.2 | 78±0.8 | 78.4±2.5 |
| Mean corpuscular hemoglobin (pg) | 24±0.7 | 27.2 | 29.1 | 23.5±0.4 | 33.7 | 33.5 | 25.7 | 25.2±1.2 |
| Reticulocyte count (%) | 1.2±0.7 | 2.1 | 0.7 | 1.1±0.4 | 1 | 0.9 | 0.9±0.7 | 0.9±0.6 |
| Leukocytes (103/μL) | 11.3±2.6 | 8.2 | 7.8 | 5.5±0.7 | 8.5 | 3.5 | 9.6±2.5 | 5.8±1.0 |
| Platelets (103/μL) | 387.7±50 | 239.5 | 233 | 273.8±26.5 | 180 | 169 | 255.5±14.8 | 312.1±63.6 |
| Hemoglobin A (%) | 38.3±1.3 | 97 | 51 | 41.0±2.2 | 52 | 95.5 | 41 | 41.4±0.9 |
| Hemoglobin S (%) | 55.2±2.1 | 0 | 45 | 54.2±1.8 | 44 | 0 | 54 | 53.6±0.5 |
| Hemoglobin F (%) | 3.5±1.0 | 1 | 2 | 2.0±0.7 | 2 | 1.5 | 2 | 1.8±0.4 |
| Hemoglobin A2 (%) | 3.0±0 | 2 | 2 | 2.8±0.4 | 2 | 3 | 3 | 3.2±0.8 |
| Alpha-globin genotype | αα/αα | αα/αα | NA | αα/αα | αα/αα | αα/αα | αα/αα | αα/αα |
Means and standard deviations refer to several values for each patient during their clinical follow-up at Hemominas Blood Center. NA: DNA not available.
All children were clinically oligosymptomatic and led normal lives. Except for an obstruction of the airways caused by adenoid glands, child number I was asymptomatic during the follow-up period. Child number II suffered from two infectious episodes: virus infection, for which a five-day hospitalization was required, and impetigo, treated with benzathine penicillin. He has had recurrent headaches, but the neurologic physical examination was normal. Except for an episode of sinusitis, child number III was asymptomatic during the follow-up period. Child number IV suffered from recurrent headaches, but the neurologic physical examination was normal. None of the children have had severe acute clinical manifestations of SCD such as acute painful crises, stroke, acute chest syndrome, or acute splenic sequestration.
DiscussionOur study described a group of Hb S/β+-Thal patients with normal or nearly normal hematological data and the absence of complications attributable to SCD. To the best of our knowledge, this is the first study to report on the occurrence of −92 (C>T), and IVS-II-844 (C>A)/IVS-II-839 (T>C) β-thal mutations in Brazil.
The degree of β-chain synthesis in Hb S/β+-Thal patients depends primarily on the β-Thal molecular mutation. The IVS-II-844 (C>A)/IVS-II-839 (T>C) mutation affects the consensus splice site and interferes with processing of the primary mRNA transcript. It modifies the conserved polypyrimidine tract of the splice acceptor site causing a reduction of beta globin chain expression to around 60% of normal.4,6 Carriers of the mutations described in this report showed a mean Hb A level of 39.7% and were oligosymptomatic. This finding is consistent with the first report of these two variants in four unrelated Canadian families of African ancestry. The probands were asymptomatic Hb S/β+-Thal patients and the adults had Hb A levels of about 44%.4 This is the second study worldwide to report these two linked sequence variants in cis. The IVS-II-844 mutation has also been identified in beta-thalassemia patients without the concomitant IVS-II-839 mutation being reported.7–9
The −92 (C>T) is a transcriptional mutation affecting the β-globin gene promoter.6 This study showed that this mutation leads to a nearly asymptomatic phenotype of SCD with a mean Hb A level of 41.4%. This finding is in agreement with those of other investigators.3 This mutation was first reported in association with the βS allele in a Sicilian adult with a high level of Hb A (45%) who was clinically asymptomatic.3 This is the second study to report the −92 (C>T) mutation in Hb S/β+-Thal patients. Heterozygous beta-thalassemia patients affected by this mutation also showed a silent form of disease, whereas compound heterozygotes with codon 39 or IVS-II-745 mutations developed beta-thalassemia intermedia.10
According to our findings and those of other investigators,3,4 both the −92 (C>T) mutation and the IVS-II-844 (C>A)/IVS-II-839 (T>C) combination cause a very mild form of Hb S/β+-Thal. These mutations do not fit into the Hb S/β+-Thal phenotypic classification as proposed by Serjeant et al. based on the amount of Hb A produced.2 This phenotypic classification was based on data from Jamaican patients with SCD and so it does not represent all Hb S/β+-Thal patients worldwide. As the original classification is limited to patients with 1–25% of Hb A, we accordingly propose here a fourth Hb S/β+-Thal phenotype, with an amount of Hb A ranging from 25 to 45%. Molecular diagnosis of these types of Hb S/β+-Thal by newborn screening programs confer a ‘good’ prognosis and will surely reduce anxiety and stress in family caregivers of children recently diagnosed with this subtype of SCD.
A few limitations of our study warrant mention. Given that the studied cases were children, possible long-term complications cannot be predicted. Owing to the small number of patients, the present report may not fully represent these rare Hb S/β+-Thal genotypes.
In summary, −92 (C>T) and IVS-II-844 (C>A)/IVS-II-839 (T>C) mutations associated with the βS allele lead to a very mild form of Hb S/β+-Thal, which we have named Type IV (25–45% of Hb A). Research is needed to determine whether other modifying genetic or environmental factors may aggravate the phenotype of affected patients.
Conflicts of interestThe authors declare no conflicts of interest.
The authors acknowledge all subjects and parents for their cooperation in the study. The research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Grant n°. 304530/2011-5), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMG, Grant n°. PPM-00266-13), Fundação Centro de Hematologia e Hemoterapia de Minas Gerais (Hemominas), and Núcleo de Ações e Pesquisa em Apoio Diagnóstico (Nupad – UFMG).