This study aims to validate the single-platform method for enumeration of CD34+ cells, by comparing the performance of two different commercial kits, as well as to evaluate the efficiency of the AccuriTM C6 cytometer in providing direct counts of absolute cell numbers.
MethodWe evaluated 20 samples from umbilical cord blood (UCB), comparing the two different methodologies for enumeration of CD34+ cells: single and dual-platform. For the assessment of the single-platform, Procount and SCE kits were used, both of which use fluorescent beads as a counting reference to obtain absolute CD34+ cells numbers. Moreover, after the acquisition of samples in flow cytometer AccuriTM C6, following the protocol established for each kit, the number of CD34+ cells was recalculated, considering the cell count provided by the AccuriTM C6.
Main ResultsIn our analysis, the results showed a strong correlation between the number of CD34+ cells/μL (r2=0.77) when comparing the SCE kit and the current dual-platform method. On the other hand, the comparison between Procount kit and dual-platform results showed a moderate correlation for the number of CD34+/μL cells (r2=0.64).
ConclusionOur results showed that the AccuriTM C6 flow cytometer can be used safely, applying both the dual and single platform analysis strategy. Considering the ISHAGE protocol-based single-platform approach, as the most appropriate methodology for CD34+ cells enumeration, our results demonstrated that the SCE kit has great potential for national standardization of UCB samples analysis methodology.
The successful use of umbilical cord blood (UCB) as a source of hematopoietic stem cells (HSCs) for transplant by Gluckman and colleagues in 1989, gave rise to the creation of Umbilical Cord Blood Banks (UCBB’s), responsible for the collection, processing and storage of UCB units.1,2
The expansion of the UCBB activity has led to the establishment of quality standards, such as those associated with the NetCord Network, the international foundation leading cord blood banks, which is currently in association with the World Marrow Donor Association (WMDA), and together they certify the largest global inventory of high quality UCB units, with more than 10,000 units already transplanted into adults and children.3
With the increase in the number of UCBB’s, precise evaluation of the number and quality of HSCs in their UCB units has become a matter of extreme importance. The number of total nucleated cells and, in particular, of HSCs, is an important selection criterion for UCB units for therapeutic use, as the number of HSCs infused is an important predictive factor of outcomes and recovery time of the hematopoietic activity after transplantation.4,5
Currently, the enumeration of these cells, based on expression of CD34 molecules on their surface, is carried out exclusively by flow cytometry.6,7 The gold standard in the enumeration of CD34+ cells was initially established in 1996 by the International Society of Hemotherapy and Graft Engineering and is known as the ISHAGE Protocol.8 This protocol determines the use of monoclonal antibodies against CD45 (pan-leukocyte) and CD34, plus analysis of lateral dispersion, or side scatter (SSC), and frontal dispersion, or forward scatter (FSC), patterns by flow cytometry. This procedure is a fast, simple and sensitive method, able to detect 10–20 CD34+ cells in every 100,000 CD45+ nucleated cells and can be applied with the use of different types of flow cytometer devices with a simple analysis algorithm.9,10
The ISHAGE protocol was initially established based on a dual-platform methodology, in which the percentage of CD34+ cells is determined by flow cytometry and the absolute leukocyte count is determined with the use of an automated hematological counter. In 1998,6 with the purpose of increasing the precision in HSCs enumeration, the following additions to the protocol were included: (1) use of fluorescent beads, in a known concentration, allowing enumeration of the absolute number of cells solely by the flow cytometric analysis; (2) use of a red cell lysis solution, without fixative agents, based on ammonium chloride (NH4Cl), and; (3) incorporation of a viability dye. The revised method allows, with the use of a single piece of equipment (single-platform), the absolute determination of the number of viable CD34+ HSCs. When analyzed in this manner, the number of CD34+ cells had a better correlation with adequate recovery of post-transplant hematopoietic activity. Since then, the revised ISHAGE protocol has been included in a variety of national and international guidelines and in several technical laboratory manuals.7,10–12
In Brazil, there is currently no standardization of CD34+ cell enumeration methodology among laboratories. However, the UCBB pursuit of quality harmonization and accreditation by certifying bodies requires the implementation of the single-platform CD34+ cell enumeration methodology for all UCB units collected by banks connected to the network. Thus, the aim of this study was to validate the single-platform methodology, comparing the results of two different CD34+ cell enumeration kits, both of which use fluorescent beads for determination of absolute cell counts, using the BD Accuri™ C6 flow cytometer. In addition, the performance of the Accuri™ C6 cytometer in providing direct counting of the absolute number of cells, excluding the use of fluorescence beads, was also evaluated.
MethodsSamplesTo validate the Accuri™ C6 system, samples were obtained from donors for allogeneic transplantation and patients for autologous transplantation, from the Brazilian National Cancer Institute (INCA). We used 65 samples from three different sources of hematopoietic stem/progenitor cells (HSPCs), namely, bone marrow (BM; n=6), mobilized peripheral blood (MPB; n=16) and UCB; n=43). Following collection, all the samples were sent to the UCBB. An aliquot of these fresh samples was sent to the Immunology Lab for enumeration of CD34+ cells by the FACScan system. This process is part of the routine laboratory analysis at the Bone Marrow Transplantation Center. After analyzing the results and releasing the CD34+ cells at the Immunology Lab, the leftover of the stained sample was sent to the UCBB for analysis by a second acquisition round reproduction of the CD34+ enumeration protocol, using the Accuri™ C6 system. Thus, the same stained sample was read on both devices, using the dual-platform ISHAGE, the same acquisition and analysis strategy.
For validation, samples included in the UCBB routine process for cryopreservation and UCB storage for transplantation were used, following the inclusion and exclusion criteria defined by the bank. Therefore, the UCB study with an initial cell count of 1.5×108 total nucleated cells (TNCs) was included in the study, excluding pregnant women with infectious diseases, identified by prenatal serology and/or clinical findings. To validate the single-platform methodology, we used 20 samples of UCB, collected from voluntary donors with due consent.
The UCB was collected by the nursing team of the UCBB-INCA at affiliated maternity hospitals or by the responsible obstetrician at non-affiliated maternity hospitals. The samples were kept at 2–8°C and transported at a temperature of 4–24°C, prior to processing for volume reduction, within 48h from collection. At the end of this process, an aliquot was sent to the Immunology Laboratory for enumeration of CD34+ cells by the dual-platform procedure currently used at the institution, using the FACScan™ flow cytometer, and concomitant enumeration of CD34+ cells by the proposed single-platform method, using the Accuri™ C6 flow cytometer at the UCBB-INCA.
Flow cytometryThe Accuri™ C6 flow cytometer is used at the UCBB-INCA for quality control of the UCB HSCs for all cryopreserved units released and selected for transplantation, as a part of the stability plan required by current norms. The dual-platform assay was performed at the Immunology Laboratory-INCA, following their routine flow cytometry standard procedure for CD34+ HSC enumeration, using the FACScan™ flow cytometer.
Single-platform: ProCOUNT kitThe ProCOUNT kit (BD Biosciences) consists of two reagent cocktails, the nucleic acid dye YO-PRO-1 and the monoclonal antibody CD34 labeled to peridinin-chlorophyll protein (PerCP), clone 2D1. The other reagent cocktail is used as a control to evaluate the amount of nonspecific binding of the PE-associated monoclonal antibody and contains a nucleic acid dye, PE-associated immunoglobulin G1 and the CD45 monoclonal antibody described above. These reagents are used in association with the Trucount BD tubes in kits containing a freeze-dried microsphere of 4.2μm fluorescent beads that dissolve by adding blood and reagents, releasing a known number of fluorescent beads.
The protocol, acquisition and analysis established by the manufacturer were followed. The acquisition strategy was to collect a minimum of 60,000 CD45 positive events and 1,000 beads, as recommended by the manufacturer.
Single-platform: SCE (stem cell enumeration) kitThe SCE kit (BD Biosciences) consists of a cocktail of reagents containing anti-CD34-PE, clone 8G12; anti-CD45-FITC, clone 2D1; a nucleic acid dye [7-aminoactinomycin-D (7-AAD)] to exclude dead cells; a lysing solution based on ammonium chloride (NH4Cl), and; BD Trucount tubes. The protocol was followed, according to the manufacturer’s instructions. The samples were acquired on the Accuri™ C6 flow cytometer immediately after the period of incubation of the lysis solution, not exceeding the maximum time limit of 1h. A positive control for 7-AAD was performed using fixed cells. For the acquisition and analysis strategies, the ISHAGE protocol was followed, with modifications associated with the addition of 7-AAD dye for the exclusion of dead cells.
Single-platform: Accuri™ C6The Accuri™ C6 flow cytometer is capable of simplifying absolute cell counting (by sample volume) by means of a microprocessor-controlled peristaltic pump system that precisely monitors the volume of the sample drawn per run. Thus, for the results for all samples acquired on the Accuri™ C6 flow cytometer, either with the ProCOUNT kit or the SCE kit, the CD34+ cell count was recalculated, taking into account the absolute cell count provided by the flow cytometer.
Dual-platformThe number of TNCs was obtained using the ABX Micros 60 hematology counter (Horiba Ltd., São Paulo, São Paulo, Brazil). In each sample, a total of 1×106 total cells was resuspended in 100μl of phosphate-buffered solution (PBS) at pH 7.2–7.4 (Sigma) with 1% bovine serum albumin (BSA) fraction V (Sigma) (PBS/BSA). Anti-human CD45-FITC (clone 2D1) and CD34-PE (clone 8G12) monoclonal antibodies were used. The cells were incubated with the specific set of monoclonal antibodies at 4°C for 20min. After this procedure, red cells were lysed with lysing solution with a fixative agent (FACS Lysing SOLUTION - BD) and incubated for 10min at room temperature (20±2°C), washed with PBS/BSA and subsequently preserved, protected from light, until the reading in PBS/1% formaldehyde. For the acquisition of samples according to the ISHAGE protocol, the strategy used was to acquire at least 75,000 CD45 positive events.
Software and statistical analysisThe BD CellQuest™ Pro and BD Accuri™ C6 analysis software were used for cytometric analysis strategies. The statistical data were analyzed using the software GraphPad Prism, version 8.0.1 (GraphPad software, San Diego, CA, USA) and EXCEL (Microsoft Inc.). A descriptive analysis was performed, including mean, standard deviation (SD), median and minimum and maximum range. The degree of linear relationship between variables was assessed by calculating the Pearson correlation coefficient.
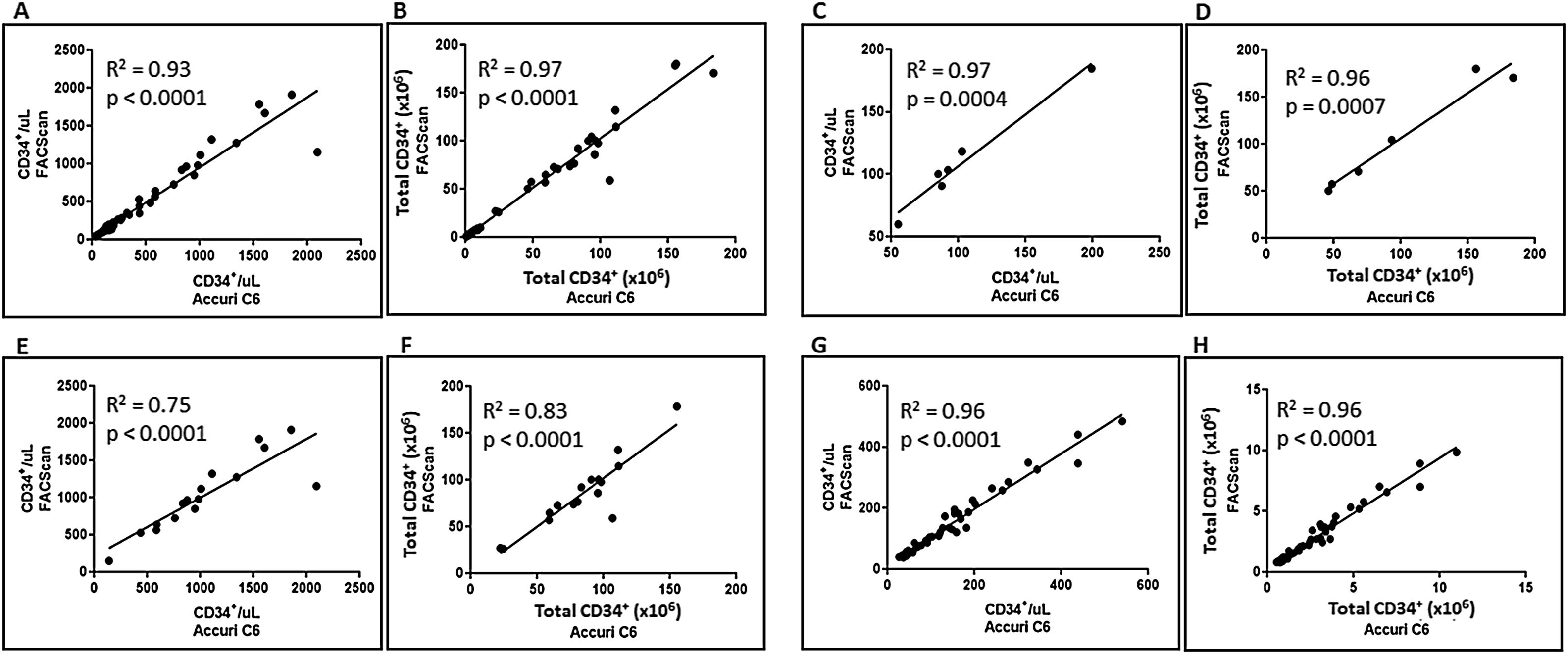
ResultsAs the Accuri™ C6 flow cytometer has not been approved by the Brazilian National Health Surveillance Agency (ANVISA) for clinical use, an initial validation of its performance for enumeration of CD34+ cells of different sources was performed, following the ISHAGE protocol for analysis. The numbers of CD34+ cells/μL and total CD34+ cells obtained with the Accuri™ C6 were compared with those obtained when the same sample was run in a device approved by ANVISA for clinical use, the BD FACScan™. As demonstrated in Figure 1, linear regression analysis showed excellent correlation coefficients for the number of CD34+ cells/μL and total CD34+ cell count in all samples, independent of the HSC source (A and B), from BM (C and D), MPB (E and F) and UCB (G and H). These results demonstrated that the Accuri™ C6 flow cytometer is equivalent to the BD FACScan™ for the quantification of CD34+ HSCs, when using dual-platform approach.
Correlation between the number of CD34+ cells/μL obtained with the Accuri™ C6 and FACScan™, of the total number of samples, independent of the HSC source (A and B), or from BM (C and D), MPB (E and F) and UCB (G and H) samples. In this interlaboratory analysis, the ISHAGE protocol was applied at both laboratories, using the dual-platform methodology.
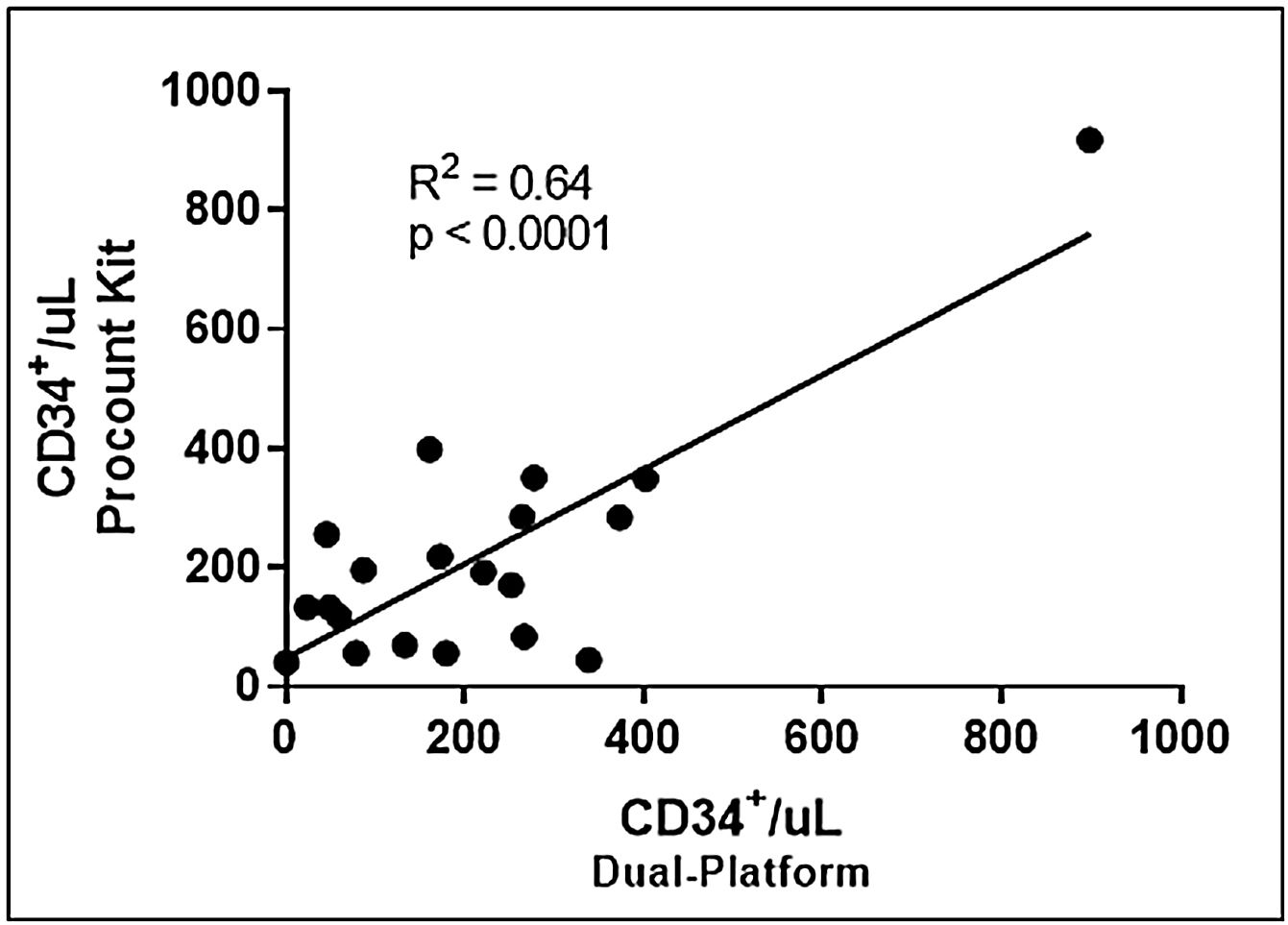
The results obtained with the standard dual-platform were compared with those obtained with the ProCOUNT Kit, as described in methods.
Linear regression analysis demonstrated a moderate correlation for the number of CD34+cells/μL (r2=0.64) obtained with the ProCOUNT kit, compared to the dual-platform methodology, as shown in Figure 2. The median values obtained were 176.5 CD34+ cells/μL (1.6–898.4cells/μL) for the dual-platform and 181.9 CD34+/μL (41.4–917.7 cells/μL) for the single-platform, ProCOUNT kit.
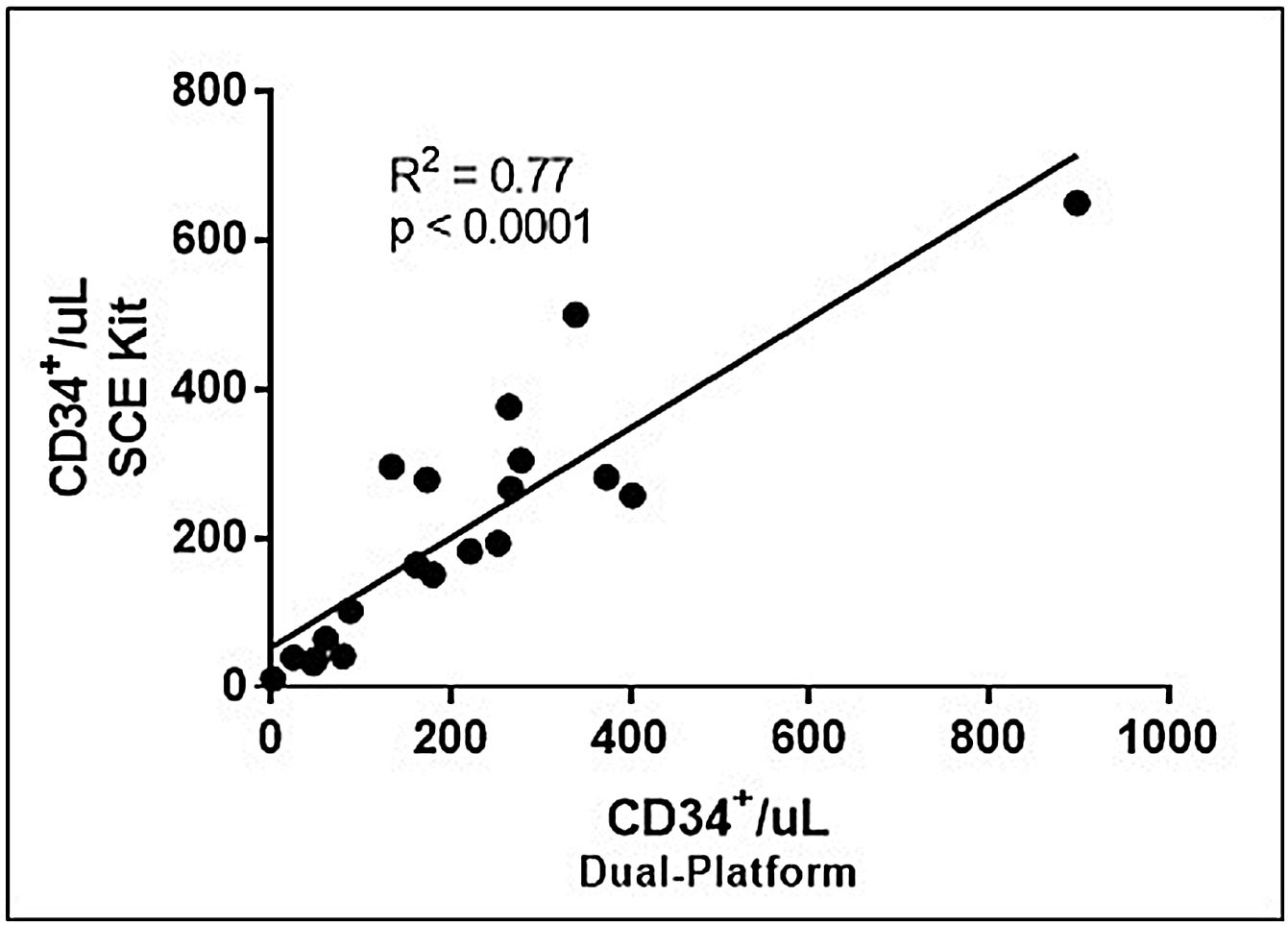
Dual vs. single-platform – SCE kitThe results obtained with the standard dual-platform were also compared with those obtained with the SCE Kit, as described in the methods.
Linear regression analysis demonstrated a strong correlation for the number of CD34+/μL (r2=0.77) obtained with the two platforms, as shown in Figure 3. For the SCE kit, the medians were 187.7 CD34+/μL (11.1–649.7cells/μL).
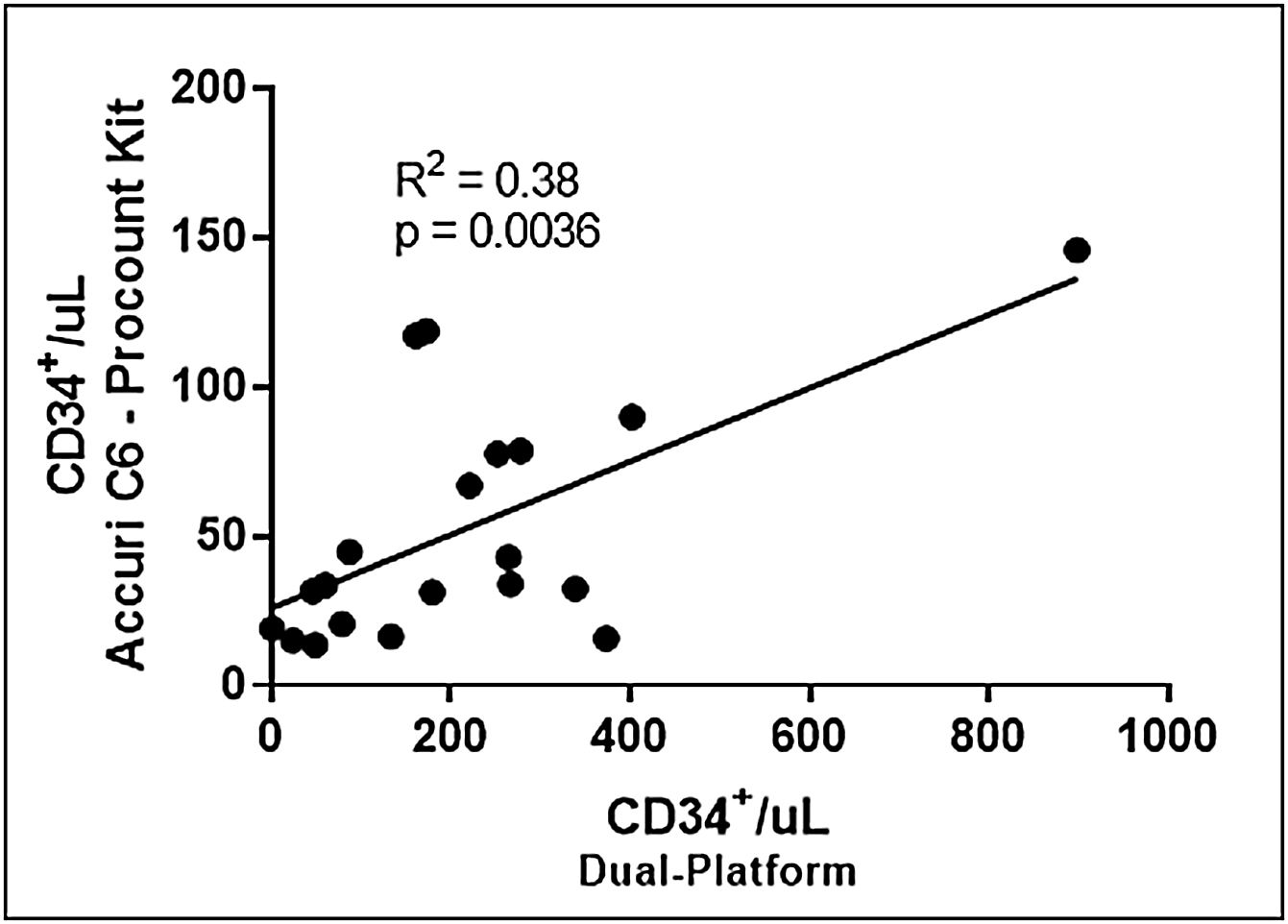
Dual vs. single-platform – Accuri™ C6Following the acquisition of the samples, either following the ProCOUNT kit protocol or that of the SCE kit, the numbers obtained were used to recalculate the CD34+ cell numbers, considering the direct cell count provided by the Accuri™ C6 equipment, instead of the counting beads. Thus, when compared to the dual-platform with Accuri™ C6, using the ProCOUNT kit protocol, linear regression analysis showed a weak correlation in the number of CD34+ cells/μL (r2=0.38), Figure 4. The median value obtained by the direct count provided by the Accuri™ C6 was 33.8 CD34+ cells/μL (13.5–145.7cells/μL).
Correlation between the number of CD34+/μL obtained by dual-platform and single-platform. The ISHAGE strategy was applied only in the dual-platform methodology. In the single-platform methodology, using the Accuri™ C6 flow cytometer and the ProCOUNT kit protocol for staining and acquisition, the absolute cell count was obtained directly from the cytometer.
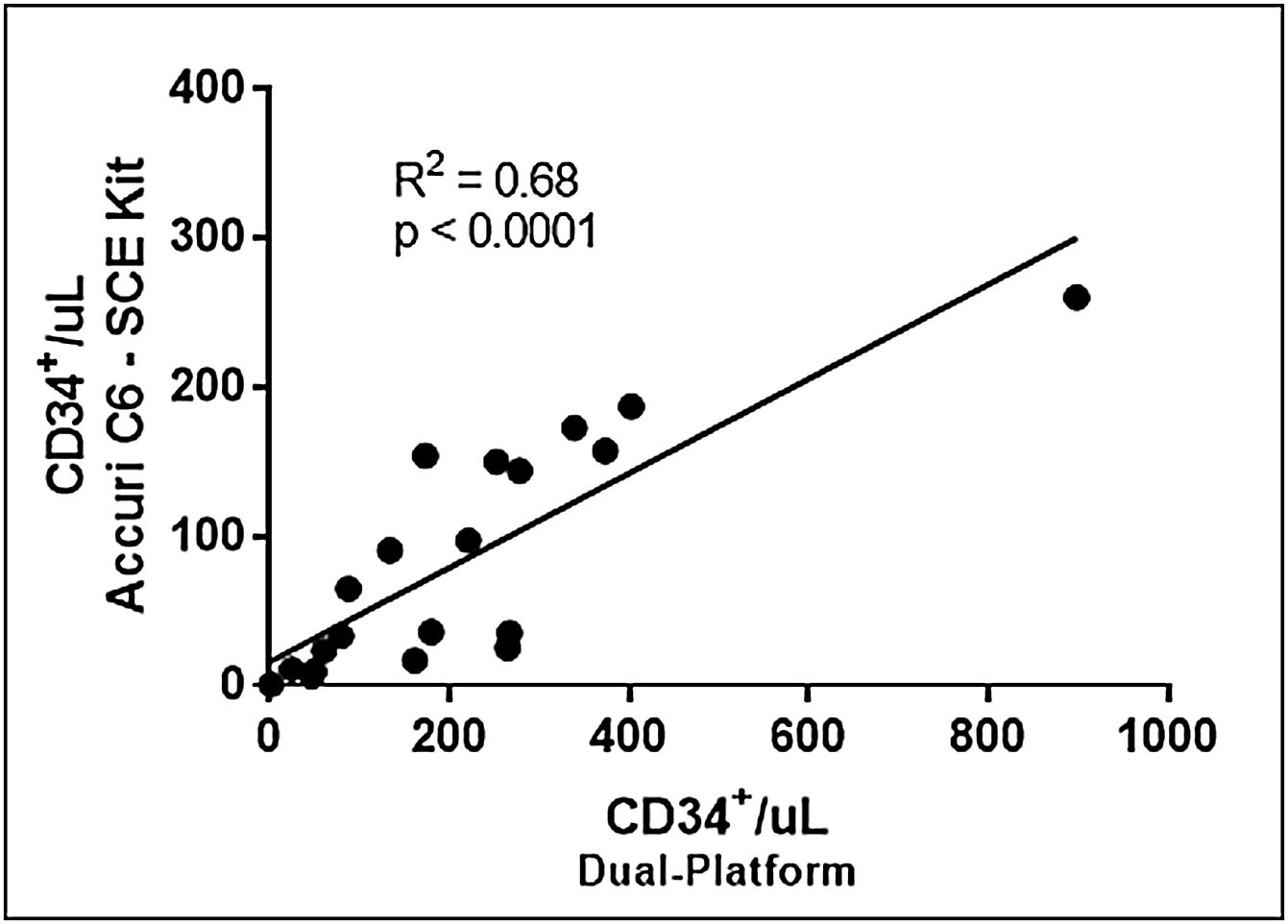
Comparing the dual-platform method with Accuri™ C6 direct counts, but using the SCE kit protocol, linear regression analysis showed a moderate correlation in the number of CD34+ cells/μL (r2=0.68), Figure 5. The median obtained by the direct Accuri™ C6 count was in this case 50.3 CD34+ cells/μL (0.7–259.8cells/μL). This result, the number of CD34+ cells/μL obtained using the SCE Kit, indicates that the Accuri™ C6 technology for direct evaluation of the absolute number of cells, in substitution of the dual-platform methodology, or the use of bead-counting for the quantification of CD34+ cells, can be considered. However, further evaluations are necessary to adapt the protocol for the ideal cell concentration range to the equipment's fluidic system.
Correlation between the number of CD34+/μL obtained by dual-platform and single-platform. The ISHAGE methodology was applied in both analyses. In the single-platform methodology, using the Accuri™ C6 flow cytometer and the SCE kit protocol for staining and acquisition, the absolute cell count was obtained directly from the cytometer.
In HSC transplantation, the quality of the graft depends on the number of CD34+ cells infused and their exact enumeration is indispensable at any facility manipulating HSC for transplantation. Given the importance of the reliable enumeration of these cells, the precision and reproducibility of their determination is crucial.13 The use of flow cytometry has become indispensable in clinical and laboratory practice for the identification and enumeration of HSCs. However, multicenter studies have shown a large interlaboratory variability in the enumeration results of CD34+ cells, which is due to many variables, such as sample origin, sample processing protocol, type of cytometer used, technical experience of the staff and type of CD34 monoclonal antibody used. The differences in the methodology employed (for instance, single- vs. dual-platform), has been shown to have a significant impact on the variability observed.14,15
CD34+ cells are considered rare. In UCB, they represent less than 1% of the total leukocytes and, given the challenges associated with the quantification of rare events by flow cytometry, the standardization of the protocols for the enumeration of these cells becomes critically necessary.9 The enumeration of CD34+ cells from UCB is critical, when compared to BM and MPB, due to the fact that UCB has additional challenging characteristics, such as: (1) a varied number of erythroblasts, which can increase leukocyte count and (2) the storage time, associated with the period between collection and processing of UCB units, which often exceeds 24h, resulting in an increased number of cells in apoptosis, dead cells and debris, factors that can lead to errors in the analysis.5
Studies have shown that the single-platform technique allows for a higher level of standardization, demonstrated by the low index of result variability in interlaboratory analysis.7,17,18 Two factors can be associated with this high level of standardization. First, the single-platform method does not consider the percentage of CD34+ cells as a ratio of leukocytes or TNCs in the sample. Second, the single-platform avoids the need for a second instrument, which in this case is the hematological counter.7
The first kit based on the single-platform methodology to be marketed was the ProCOUNT kit, in 1994.19 Initially, the assay was optimized only for peripheral blood (PB) or MPB samples, not being indicated for UCB or BM samples, and was not used to evaluate cell viability or for subpopulation analysis.5 In 1998, Hübl et al.20 described modifications in the ProCOUNT assay so that it could be applied in UCB samples. Modifications included replacing the nucleic acid dye with a viable dye, YO-PRO-1 (FL1 channel) and the threshold becoming defined in the CD45 (FL3 channel) label. However, the need for manual adjustments in the analysis regions for each sample, which is still required, reduces the potential of the assay, as such adjustments require an experienced cytometrician.
In 1996, in a multicenter study, Chang and Ma,18 demonstrated that the choice of analysis strategy had a greater impact on the enumeration of CD34+ cells and that the standardization allowed by the ISHAGE protocol provided reproducible results.
In 2005, Humpe et al.21 observed a significant difference in the viability of total CD45+ and CD34+ cells after cryopreservation and attributed this fact to the higher resistance of CD34+ cells, when compared to the more differentiated cells, specifically neutrophils, after thawing. Therefore, differences associated with the use of fresh/frozen samples, which are particularly relevant in the UCBB activity, adds an additional layer of complexity to the protocol standardization.
The addition of fluorescent beads and viability dye to the ISHAGE protocol allowed for its conversion into a single-platform-compatible methodology.6 Attentive to the customer needs, BD Biosciences developed a new kit based on this new technology, the SCE kit. In 2009, Sutherland and colleagues10 established the use of the SCE kit in PB and MPB samples, comparing two flow cytometers, FACSCalibur™ and FACSCanto™. The authors demonstrated the efficacy of the SCE kit in both cytometers, obtaining a high reproducibility in the CD34+ cell enumeration for the types of samples studied.
In 2011, Dauber et al.22 evaluated the use of the SCE kit in PB, MPB, BM and UCB samples before and after cryopreservation and concluded that the SCE kit, in addition to complying with all clinical requirements, is a single-platform, flow cytometric assay, which generates fast and reliable results for the number of viable HSCs of different origins.
Although both kits used in the study are well standardized for the quantification of CD34+ cells, the ISHAGE protocol has been considered the gold standard for the quantification of these cells, as the SCE kit has an advantage over the Procount kit. Our results demonstrated a better correlation between the number of CD34+/μL cells (r2=0.77) with the use of the SCE kit, when compared to the Procount kit (r2=0.64) or the current standard dual-platform method.
Both kits evaluated in this study were accompanied by Trucount BD tubes containing lyophilized fluorescent beads microspheres that are used to evaluate the ratio of beads to CD34+ cells to obtain the absolute number of CD34+ cells.7 However, BD has launched the Accuri™ C6 flow cytometer that simplifies cell analysis by allowing direct counting.
In our analysis, the difference between the hematological counter and the direct cytometric count was 24% for the samples acquired by the ProCOUNT kit and 31% in the samples acquired by the SCE kit. Based on the manufacturer's indication that the Accuri C6 provides a reliable total nucleated cell count (TNC), we recalculated the number of CD34+ cells based on the TNC count directly from the Accuri C6 cytometer to replace the hematological counter. However, the TNC numbers used in the tests were based on the criteria established by the manufacturer for each kit (ProCount x SCE), which is superior to that established by the Accuri C6 Manufacturer to provide a reliable count. In the study, the concentration of cells used showed an average of 23.6×106cells/mL, according to the indication of each kit, with a maximum concentration of up to 50×106cells/mL for the ProCOUNT kit and 40×106 cells/mL for the SCE kit. Therefore, the high concentration may have resulted in inaccurate counts due to system saturation.
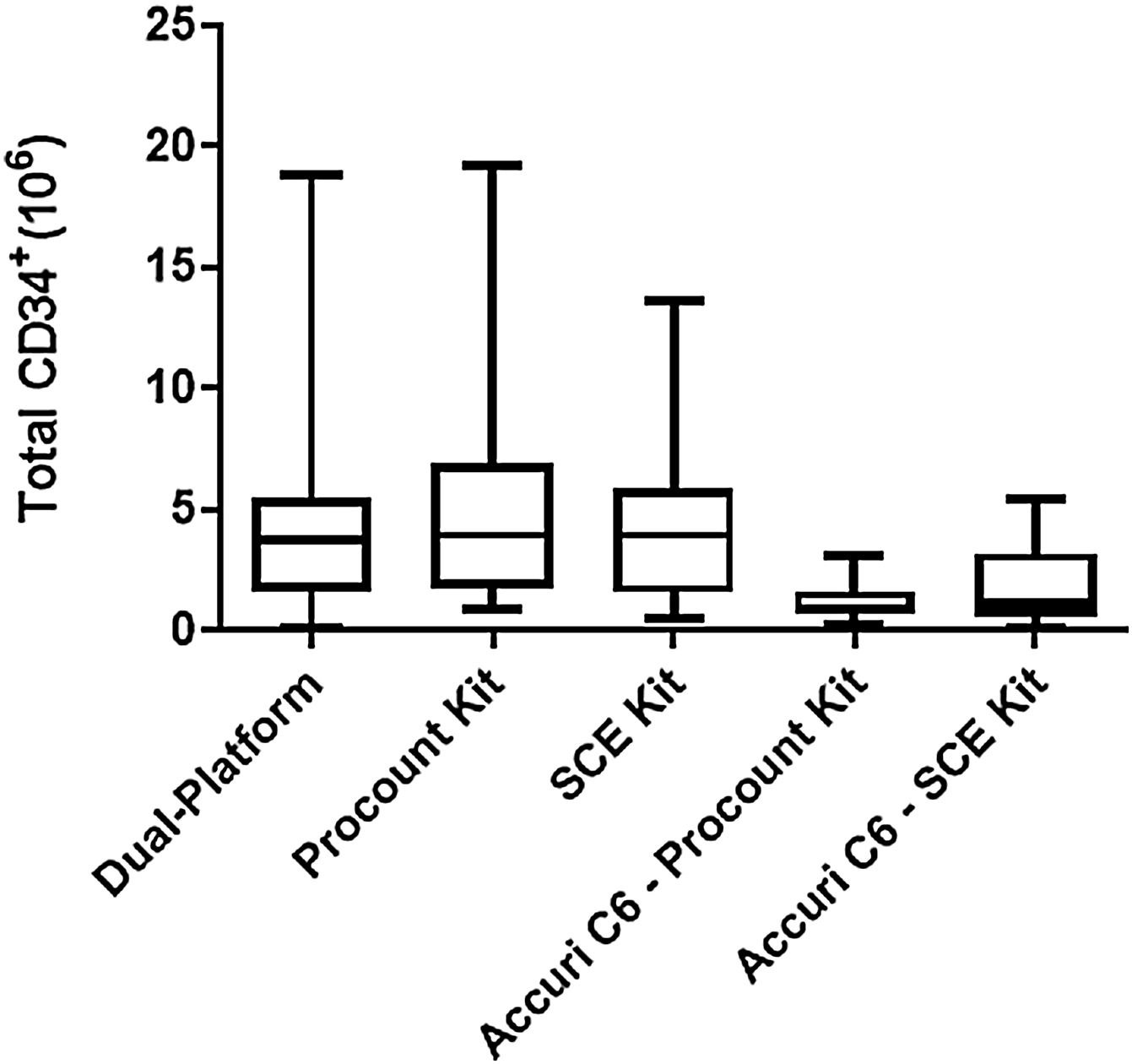
In relation to the total number CD34+ cells, when comparing the single-platform with the dual, both SCE and ProCOUNT Kits had close medians and although the SCE kit had less dispersion, both kits can be considered for replacing the dual-platform analysis at the UCBB-INCA (Figure 6 and Table 1). It is important to recognize that, in the case of UCB units, the accuracy of CD34+ cells enumeration is critical. An overestimation of the number of CD34+cells would increase the risk of graft failure when the unit is used for transplantation, while an underestimation could lead to exclusion of a UCB unit with potential for transplantation.
Evaluation of the median number of total CD34+ cells obtained with the different methodologies. The median obtained with SCE kit was closer to that obtained by the current dual-platform method. The results obtained by the Accuri™ C6 were lower than those obtained with the dual-platform method.
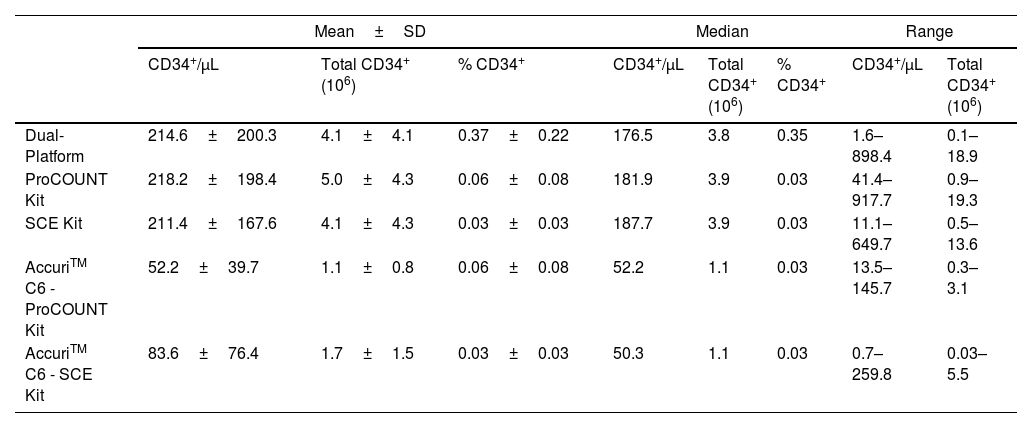
Descriptive statistical analysis, including mean, standard deviation (SD), median and range of minimum and maximum values of the different CD34+ cell enumeration methodologies.
| Mean±SD | Median | Range | ||||||
|---|---|---|---|---|---|---|---|---|
| CD34+/μL | Total CD34+ (106) | % CD34+ | CD34+/μL | Total CD34+ (106) | % CD34+ | CD34+/μL | Total CD34+ (106) | |
| Dual-Platform | 214.6±200.3 | 4.1±4.1 | 0.37±0.22 | 176.5 | 3.8 | 0.35 | 1.6–898.4 | 0.1–18.9 |
| ProCOUNT Kit | 218.2±198.4 | 5.0±4.3 | 0.06±0.08 | 181.9 | 3.9 | 0.03 | 41.4–917.7 | 0.9–19.3 |
| SCE Kit | 211.4±167.6 | 4.1±4.3 | 0.03±0.03 | 187.7 | 3.9 | 0.03 | 11.1–649.7 | 0.5–13.6 |
| AccuriTM C6 - ProCOUNT Kit | 52.2±39.7 | 1.1±0.8 | 0.06±0.08 | 52.2 | 1.1 | 0.03 | 13.5–145.7 | 0.3–3.1 |
| AccuriTM C6 - SCE Kit | 83.6±76.4 | 1.7±1.5 | 0.03±0.03 | 50.3 | 1.1 | 0.03 | 0.7–259.8 | 0.03–5.5 |
The cost/benefit ratio of each methodology must be evaluated according to the reality of each center. Considering the inputs per test, following the use of reagents as recommended by the manufacturer, the double platform is approximately 30% cheaper than the simple kit-based platform, using Trucount tubes. Compared to the dual platform, the simple platform allows results to be released in less time, requires less manipulation during cell marking, having less risk of cell loss during the development of the protocol, as it does not require washing, and greater consistency in interlaboratory results. The latter is particularly important in the routine of releasing products for transplantation from centers connected in networks.
For UCBB activities, particularly in regard to the assessment of the inventory stability plan, a simple platform for the quantification of viable HSCs is necessary, as the TNC profile after thawing is different, as granulocytes are the first cells to die after the cryopreservation process, which leads to an imprecision in the quantification result of CD34+ cells in the double platform methodology, making it impossible to evaluate the recovery of these cells during the storage period, impairing the qualitative analysis of the inventory.
ConclusionOur results showed that the Accuri™ C6 flow cytometer can safely be used, applying both the dual and single platform analysis strategy. Considering the single platform approach based on the ISHAGE protocol as the most appropriate methodology for the enumeration of CD34+ cells, our results demonstrated that the SCE kit has great potential for national standardization of the UCB sample analysis methodology before and after thawing, allowing for a reliable analysis of post-thaw recovery of CD34+ cells and, in accordance with current standards, allowing for the implementation of a fully validated and standardized approach for the stability analysis of the UCBB national inventory.
Conflicts of interestThe ProCOUNT and SCE kits used in the study were provided by BD Bioscience. The authors are responsible for the written content in this study and data analysis. The authors declare they have no financial interests.
The authors wish to thank the Brazilian Ministry of Health and the Pro-Vita Association for their continued support to the Umbilical Cord Blood Program in Brazil, as well as the National Bank for Economic and Social Development for supporting the continued improvement and expansion of the Brazilian Network of Umbilical Cord Blood Banks, which enabled the acquisition of the equipment used in this study.