Flow cytometry has become an increasingly important tool in the clinical laboratory for the diagnosis and monitoring of many hematopoietic neoplasms. This method is ideal for immunophenotypic identification of cellular subpopulations in complex samples, such as bone marrow and peripheral blood. In general, 4-color panels appear to be adequate, depending on the assay. In acute leukemias (ALs), it is necessary identify and characterize the population of abnormal cells in order to recognize the compromised lineage and classify leukemia according to the WHO criteria. Although the use of eight- to ten-color immunophenotyping panels is wellestablished, many laboratories do not have access to this technology.
Objective and MethodIn 2015, the Brazilian Group of Flow Cytometry (Grupo Brasileiro de Citometria de Fluxo, GBCFLUX) proposed antibody panels designed to allow the precise diagnosis and characterization of AL within available resources. As many Brazilian flow cytometry laboratories use four-color immunophenotyping, the GBCFLUX has updated that document, according to current leukemia knowledge and after a forum of discussion and validation of antibody panels.
ResultsRecommendations for morphological analysis of bone marrow smears and performing screening panel for lineage (s) identification of AL were maintained from the previous publication. The lineage-oriented proposed panels for B and T cell acute lymphoblastic leukemia (ALL) and for acute myeloid leukemia (AML) were constructed for an appropriate leukemia classification.
ConclusionThree levels of recommendations (i.e., mandatory, recommended, and optional) were established to enable an accurate diagnosis with some flexibility, considering local laboratory resources and patient-specific needs.
The Brazilian Group of Flow Cytometry (Grupo Brasileiro de Citometria de Fluxo, GBCFLUX) had previously proposed monoclonal antibodies (MoAb) panels to be subsequently validated in an interlaboratory study to assess their effectiveness in the diagnosis and classification of acute leukemia (AL).1 The new flow cytometers (CF) allow immunophenotyping using 8 or more colors,2-4 however, many Brazilian laboratories still work on 4-color multi-parameter flow cytometry (MCF) platforms. The present document aims to update the former reported in 2015.1 The panels previously proposed were revised, based on a literature review and the extensive experience of the professionals participating in this study. The former goals of the panels were maintained: 1) identification and quantification of abnormal leukemia cells, as well as lineage identification by the screening tubes; 2) disease classification according to the cell maturation stage; 3) identification of leukemiaassociated immunophenotypes (LAIPs) to be used for minimal residual disease (MRD) assessments, and; 4) identification of phenotypes associated with molecular alterations with well-recognized prognostic implications.1-5
All the panels were designed at different recommendation levels for diagnosis and classification, allowing flexibility compatible with the local laboratory resources. Three levels of recommendations were considered: mandatory, recommended and optional. Mandatory recommendations contain the minimum criteria for identification, quantification and classification of AL. Recommended level includes markers that are not essential for diagnosis, but are important for leukemia subclassification, prognosis and MRD detection. Optional recommendations include markers useful for MRD evaluation, detection of less frequent leukemia subtypes, associated with molecular or cytogenetic abnormalities, and prognosis, such as CD66c, CD123 and NG2.2 These panels were validated in the group's laboratories to assure their effectiveness (see supplementary files).
General recommendationsThe recommended pre-analytical and analytical processes have been previously described.1,6
Detailed patient information is essential for initial evaluation and should contain age, gender, complete blood cell counts, disease phase (diagnosis and monitoring), previous treatment and whether it is a relapse or a secondary transformation. In addition, cytomorphology information is useful as a complementary method to flow cytometry for the appropriate diagnostic assessment of AL. It is therefore also desirable to have information about bone marrow (BM) cytology and a description of the blast cells.
However, the cell morphology analysis should not be used as a screening panel substitute, only as additional diagnostic information.
Although this document intends to standardize the approaches for the diagnosis of acute leukemia AL in 4-color MFC, the GBCFLUX Committee for Acute Leukemia recommends that Brazilian flow cytometry laboratories, when possible, migrate to platforms of 8 or more colors for a more accurate diagnosis and the use of more sensitive methods for MRD detection.
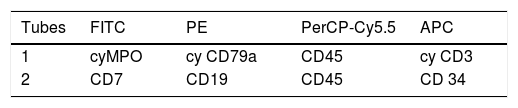
Acute leukemia screening tubes (ALST)Two tubes are designed to lineage identification of the leukemic blast: B-cell lineage (CD19 and cyC79a), T cell lineage (cyCD3), myeloid (cyMPO) and ambiguous lineage leukemia (Table 1). In addition, some markers were added to improve blast cell identification, such as CD34 as an immaturity marker, CD7, which is very frequent in T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) and in some acute myeloid leukemia (AML), and CD45, that can be used as auxiliary marker for the gating strategy. Compared to the original publication, the 1st tube was maintained and the 2nd tube was slightly modified, with a switch of the CD7 and CD19 fluorochromes. The CD7 is a strong marker and has been combined with fluorescein isothiocyanate (FITC), according to the classic criterion that highlyexpressed markers must be conjugated with a weak fluorochrome.2,6,7 This is a general rule for choosing MoAb and fluorochromes for a panel. For the same reason, we recommended the CD19 conjugated with phycoerythrin (PE): low density markers should be conjugated with bright fluorochromes.6,7
Based on the immunophenotypic information derived from the ALST tubes, lineage-directed panels [B-cell precursor acute lymphoblastic leukemia/lymphoma (BCP-ALL) or T-lymphoblastic leukemia (T-ALL) or acute myeloid leukemia /myelodysplastic syndrome (AML/MDS)] must be applied in order to provide the final diagnosis (see Tables 1, 2 and 3).
BCP-ALL Antibodies Panel for 4-Color Immunophenotyping – markers and fluorochromes.
FITC: fluorescein isothiocyanate; PE: phycoerythrin; PerCP-Cy5.5: peridinin chlorophyll protein/cyanin5; APC: allophycocyanin; Cy: cytoplasmic; Nu: nuclear; Sm: surface membrane* according to expression in the screening panel.
BCP-ALL molecular abnormalities and related immunophenotypic profiles.a
The ALST should be used in all suspected cases of AL, even in those with typical cytomorphological findings, in order to identify the AL lineage and also the ambiguous lineage phenotype (Table 1). However, the screening tube is not enough to reach the final diagnosis. On the other hand, the lineage of some subtypes of leukemia, such as megakaryoblastic leukemia, AML with minimal differentiation, blastic plasmacytoid dendritic cell neoplasm, as well as acute undifferentiated leukemia, cannot be defined by the ALST tube. These AML subtypes often do not express intracytoplasmic myeloperoxidase (cyMPO) nor the lymphoid markers that define the B and T lineage. Thus, in any situation, an expanded assessment should be performed to accurately define the leukemia classification. The rationale for choosing ALST markers has already been described1.
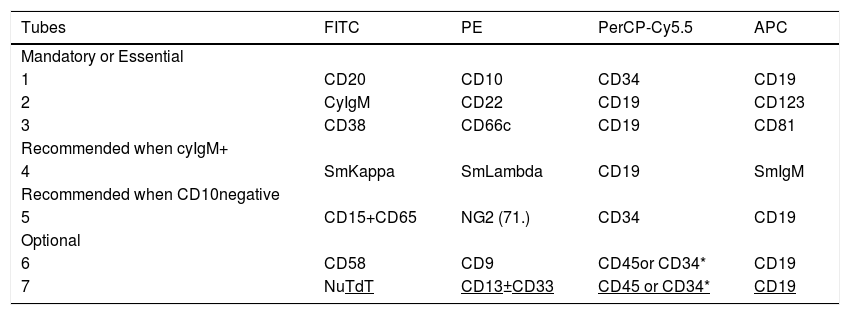
Classification panel for B cell precursor acute lymphoblastic leukemia/lymphomaBCP-ALL are immature B-cell malignancies involving bone marrow (BM), peripheral blood (PB) and even presenting primary involvement of nodal or extra nodal sites (BCP-LBL). The BCP-ALL immunophenotypically is diagnosed by the differences between the patterns of antigen expression in leukemic cells and their normal counterparts. As a result, the CD19 has been used as a backbone marker of B-cell lineage. Tubes 1 to 4 allow the assessment of B-cell maturation and can detect leukemia cells by "different from normal" cell patterns. The BCP ALL is diagnosed phenotypically by the differences in the constitution of the tubes, compared to the previous recommendations described below (Table 2).
In mandatory tubes, which include markers that allow the identification of normal maturation and asynchronous antigens expression of B cells (Table 2: tube 1), the CD34 FITC, present in the previous combination1 was changed to PerCP-Cy5.5, due to the better performance of this marker in this fluorochrome. In BCP-ALL, the CD38 and CD81 MoAb usually show expressions unlike normal B cell maturation, which is easily identifiable, as well as useful for detecting MRD6.
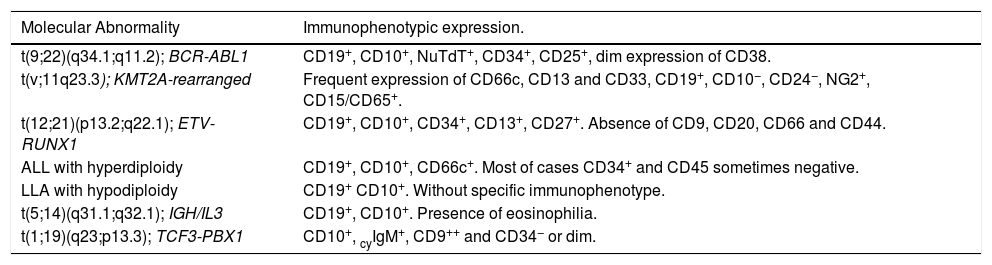
The rationale for the CD22 in the panel is its usefulness in evaluating the use of anti-CD22 therapy, according to its intensity of expression. It is also useful to replace the CD19 as a B cell marker for MRD detection after anti-CD19 immunotherapy.8 In addition, cross-lineage markers, such as CD66c and CD123, were included as mandatory markers instead of CD13 and CD33 from the previous panel.1 The CD66c and CD123 are more informative for molecular lesions (BCR-ABL fusion gene and hyperdiploidy)5,9,10 and both are more frequently expressed and stable after ALL treatment than the CD13 and CD33, being more useful for further MRD detection.6,11,12
Tube 4 is a complement to tubes 1, 2 and 3 for the classification of ALL, based on the stage of maturation, allowing the diagnosis of mature B-cell leukemia.13 This tube is recommended to be used in combination with the others, because the identification of mature B-cell ALL (CD20+) has therapeutic implications (for example: use of anti-CD20 associated with chemotherapy).14
Tube 5 is recommended for all infants and children with ALL CD10 negative. It is not optional, as in our previous guidelines, because CD10 negativity, added to the expression of NG2, CD15 and CD65 are generally associated with KMT2A rearrangements (MLL), which are more common in pediatric patients.15,16
In addition, the optional tube 6 includes the CD9 and CD58, which provide additional diagnosis information. The CD58 is an interesting LAIP marker since it is highly expressed in malignant B-cells, but found at low levels in normal/regenerating B-cell precursors and mature B-cells.17,18
Tubes 6 and 7 include CD45 or CD34 with CD19, according to expression in the screening panel. The choice between the two markers depends on the most informative antibodies in the previous tubes of the panel.
The NuTdT, CD58, CD13 and CD33 are markers that can provide some additional information, but the most important markers for these two tubes are the CD9 and CD25.19,20 We recommend the use of the CD9 instead of CD25 in the 2nd fluorescence if the phenotype suggests: 1) t (1; 19) or rearrangement TCF3-PBX1, that is, pre-B ALL phenotype with homogeneous CD10 and CD19 and partially positive CD20 with strong positive CD92 if the phenotype suggests t (12; 21) or fusion gene ETV6-RUNX1, that is, a common ALL phenotype, with partial or total loss of CD9.21 On the other hand, we recommend the use of the CD25 if the phenotype suggests Ph + ALL: common B-ALL phenotype with strong homogeneous CD10 and CD34, heterogeneous CD38 (positive to negative) and CD66c positive.2,19-20
Table 3 shows the BCP-ALL molecular abnormalities and related immunophenotypic profiles.22
The performance of the B-ALL diagnostic panel can be seen in figure 1S (supplementary files).
Classification panel for T lymphoblastic leukemia/lymphomaT-lymphoblastic lymphoma (T-LBL), which is biologically similar to T-acute lymphoblastic leukemia (T-ALL), is derived from immature lymphoid cells of T-cell lineage.23 Bone marrow involvement < 25% BM blasts are classified as T-LBL, while patients with ≥ 25% BM blasts are diagnosed with T-ALL.22 Early T-cell precursor lymphoblastic leukemia (ETP-ALL) is considered a high risk subtype of T-cell ALL/LBL (T-ALL/LBL) by the 2017 WHO classification of hematopoietic neoplasms.22
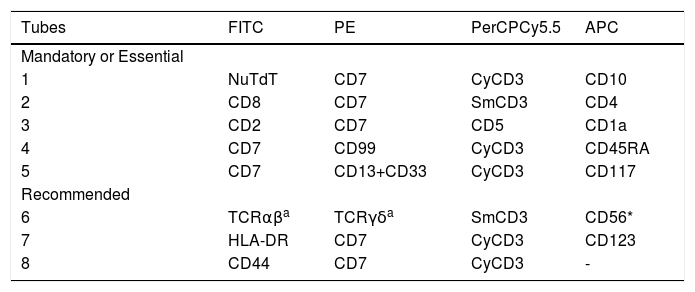
The differences in the constitution of the tubes, compared to the previous recommendations,1 are described below (Table 4).
T-ALL/LBL Antibodies Panel for 4-Color Immunophenotyping – markers and fluorochromes.
| Tubes | FITC | PE | PerCPCy5.5 | APC |
|---|---|---|---|---|
| Mandatory or Essential | ||||
| 1 | NuTdT | CD7 | CyCD3 | CD10 |
| 2 | CD8 | CD7 | SmCD3 | CD4 |
| 3 | CD2 | CD7 | CD5 | CD1a |
| 4 | CD7 | CD99 | CyCD3 | CD45RA |
| 5 | CD7 | CD13+CD33 | CyCD3 | CD117 |
| Recommended | ||||
| 6 | TCRαβa | TCRγδa | SmCD3 | CD56* |
| 7 | HLA-DR | CD7 | CyCD3 | CD123 |
| 8 | CD44 | CD7 | CyCD3 | - |
FITC: fluorescein isothiocyanate; PE: phycoerythrin; PerCP-Cy5.5: peridinin chlorophyll protein/cyanin5; APC: allophycocyanin; cy: cytoplasmic; Nu: nuclear; Sm: surface membrane.
The immunophenotypic criteria that are useful in the diagnosis of T-cell neoplasms include the absence, under expression and overexpression of one or more of the pan-T antigens (CD2, SmCD3 and CD5), in addition to the expression of anomalous and cross-lineage antigens.22 The cytoplasm CD3 and/or CD7 has been used as a backbone marker for T- cell lineage.2 Tubes 1 to 3 (Table 3) allow the classification of T-ALL according to the maturation profile of leukemic cells. Tube 4 was designed for later detection of MRD: 1) the CD99 expression is very frequently expressed and recognizes more immature T-ALLs, in addition to being stable after treatment and useful for MRD detection,2,24 and; 2) the CD45RA is expressed only in more immature subtypes of T-ALL and can be useful for detecting MRD.25 Tube 5 is essential for the identification of ETP-ALL, whose characteristics are the co-expression of cyCD3+ and CD5− / low with stem cell markers (CD34 and CD117) or myeloid markers (CD13 and CD33), in the absence of CD1a and CD8 expressions.26,27
Tube 6 is useful for subclassifying T-ALL according to TCR expressions2,28, as well as for diagnosing the precursor NK-ALL / LBL, which is the CD2+, CD7+, CD56+, and even cyCD3+, without expressions of markers from other lineages.2,22 In tubes 7 and 8: the CD123 is a cross lineage marker2,29; the HLA-DR negativity is characteristic of T-ALL,2,22 and; the CD44 is an optional marker for MRD detection. The CD44 upregulation may be involved in T-ALL leukemogenesis30 and it has been reported to be highly expressed in pediatric T-ALL.31,32 Therefore, these markers may provide additional information for the diagnosis of T-ALL.
In comparison with the first guideline, the fluorochromes CD1a, CD2, CD3, CD4 and CD5 were changed, and CD3 and CD7 were maintained at the same fluorescence in the different tubes as backbone markers to standardize the selections of the gates and to minimize the cost of the panels because these markers are in accordance with the previous recommendations for the MRD panels.6 The performance of the T-ALL diagnostic panel can be seen in Figure 2S (supplementary files).
Classification panel for acute myeloid leukemiaAcute myeloid leukemia (AML) is classified by the WHO into different categories: AML with recurrent genetic abnormalities; AML with myelodysplasia-related changes, therapy-related myeloid neoplasms; not otherwise specified (NOS), and; myeloid sarcoma and myeloid proliferations associated with Down syndrome.22Most of them have immunophenotype-associated profiles, diagnosed by multiparametric flow cytometry.22,33
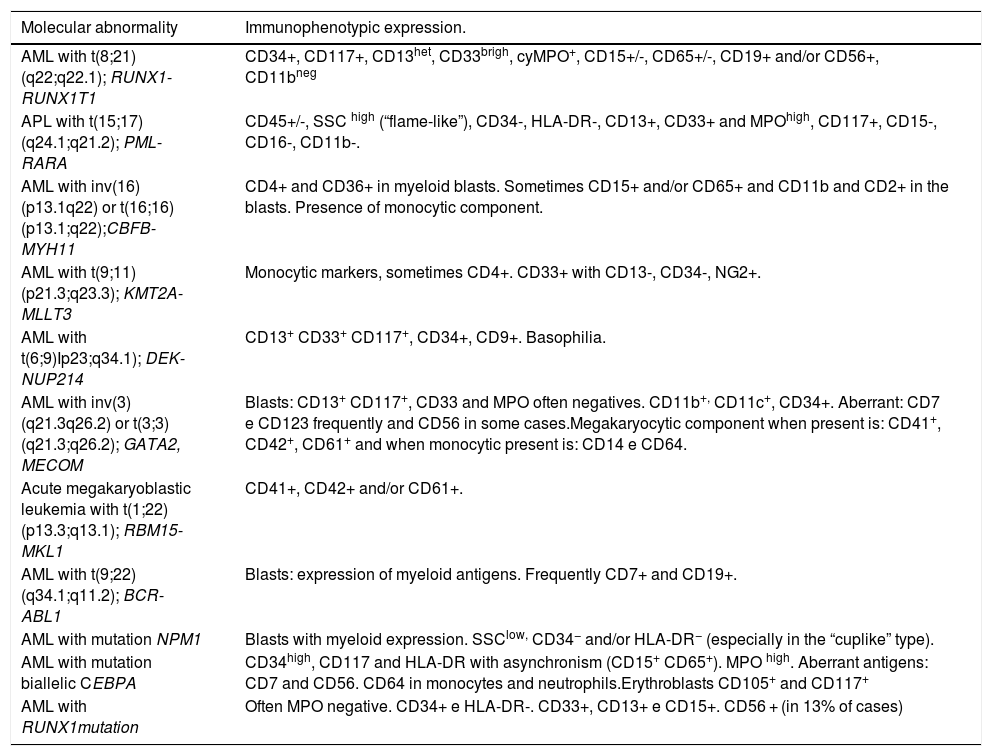
The development of AML from stem cells with specific founder mutations leads to an oligoclonal disease that progresses into a very heterogeneous leukemia at diagnosis.34,35Table 5 provides information on molecular abnormalities in AML and associated immunophenotypes.34 The immunophenotypic alterations observed in AML are asynchronous antigen expression, antigens under- or over-expression and aberrant /cross lineage marker expression.36,37
AML molecular abnormalities and related immunophenotypic profiles.a
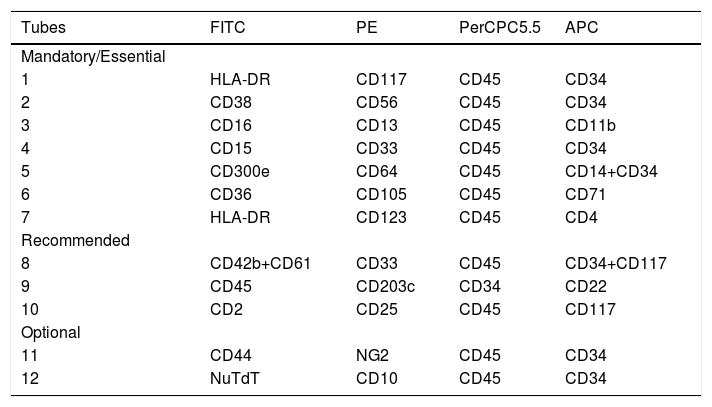
Compared to previous recommendations, a few changes have been made. The mandatory panel contains several markers that will contribute to the identification and quantification of blast cells, as well as allow classification according to the lineage differentiation or non-differentiation.
Tubes 1 and 2 (Table 6) allow the characterization of more immature myeloid compartments, including the pre-leukemic stem cells CD34+/CD38−, which may be responsible for the leukemia recurrence. Therefore, these cells should also be evaluated in the MRD assays.38–40 The second tube also includes CD56 as a cross-lineage marker, which can be useful in detecting MRD. Other cross-lineage markers are CD7, CD19 and cyCD79a, which are contained in the screening panel, and CD2, contained in tube 10 (Table 6).36,37
Monoclonal antibodies combinations recommended for the diagnosis of AML
FITC: fluorescein isothiocyanate; PE: phycoerythrin; PerCP-Cy5.5: peridinin chlorophyll protein/cyanin 5; APC: allophycocyanin.
Tubes 3 to 6 (Table 6) are designed to identify the leukemia committed lineage: neutrophil (3 and 4), monocytic (4 and 5) and erythroid (6)
Originally, tube 4 contained CD61 in the 2nd fluorochrome (FL), but this marker was replaced by CD33, which together with the other markers in tubes 3 and 4 (CD11b, CD13, CD15), allows for a better differentiation between leukemic blasts from the neutrophilic and monocytic lineage, including Acute Promyelocytic Leukemia (APL).
In tube 5, designed for assess monocytic maturation (CD64, CD36, CD14 and IREM2), CD36 was replaced by CD300e (IREM2), which can distinguish the more mature monocytic compartment (CD14+/CD300e+) from the promonocytes (CD14+/CD300e−). It is a useful combination for subclassifying monoblastic and monocytic leukemias.41 In addition, CD34 was added to CD14 in the 4th FL to identify more immature monocytic precursors and asynchronisms of antigen expressions in this lineage.
In tube 6, designed for erythroid lineage evaluation (CD71, CD36 and CD105), the glycophorin marker present in the first guideline was removed because it did not offer additional information. The CD105 (endoglin) is expressed in the early stages of erythroid differentiation (CD117+/CD45++/CD34−/CD13−/HLA-DR− cells), remains present after the levels of CD71 and CD36 increase and drops gradually after CD117 is lost, so that more mature red cell precursors no longer express CD105.1,2,42
The last mandatory tube (tube 7) is useful for the diagnosis of blastic plasmocytoid dendritic cell neoplasm (BPDCN).43,44Although this is a less frequent type of leukemia, its correct diagnosis is mandatory due to the severity of the disease and the therapeutic impact of this diagnosis. The CD4 included in this tube is useful for the diagnosis of BPDCN43 and is also expressed in monocytic leukemia.2 Furthermore, it is a cross-lineage marker that can be useful in the detection of the MRD.36
Tubes 8 to 10 are recommended in cases where the tubes described above are not able to subclassify the AML.
The recommended tube 8 corroborates the identification of blast cell involvement with the megakaryocytic lineage, as acute megakaryoblastic leukemia can be positive for CD61, CD41 and CD42.2,45 The CD41 recommended in the first guideline was replaced by CD42a plus CD61FITC due to the higher frequency of these markers in this leukemia subtype.2
Tubes 9 and 10 are intended for the diagnosis of acute basophilic leukemia and mast cell diseases respectively, including mast cell leukemia.2,46,47 The changes in the fluorochromes of CD22 and CD45 in tube 9 were justified by the better performance of CD22 in APC than in FITC and the good performance of CD45 in FITC. In addition, in tube 10 there are the CD2 and CD25 which can be useful markers for MRD purposes and prognosis in AML.48–50
Optional tube 11 contains markers to detect leukemic stem cells (CD44)38,39 and immunophenotype associated with KMT2A(MLL) rearrangements (NG2).51,52
Currently, the optional tube 12 included here is useful in the following circumstances: i) the TdT expression in myeloid and lymphoid precursors, ii) for the evaluation of lymphoid precursor cells (type I), and; iii) the CD10 expression in granulocytic cells in MDS. The CD10 and TdT unrelated to the lymphoid lineage are also good markers for detecting MRD.36,37
The AL screening and AML panel include several markers that detect the aberrant expression of lymphoid-associated antigens (CD19, cyCD79a, CD7, CD2, CD4 and CD56) and asynchronous antigen expression (CD14, CD15, CD16 and CD11b). Some of them can be useful in further MRD assessment.36,37,53 The performance of the AML diagnostic panel can be seen in Figure 3S (supplementary files).
ConclusionIn summary, this document presents updated guidelines for the use of validated 4-color panels considered relevant in the diagnosis of acute leukemia. The GBCFLUX Acute Leukemia Committee has revised the monoclonal antibody consensus panel to provide a well-defined diagnosis of acute leukemia for clinical flow cytometry laboratories, including those with limited resources. This technical standardization should improve the quality of the acute leukemia diagnostics among different laboratories. In addition, this work paves the way for multicenter cooperative studies, promoting scientific and technological advances in clinical flow cytometry in Brazil.
Finally, these diagnostic guidelines can be replicated in laboratories that still use 4-color cytometers until they migrate to the 8- to 12-color ones.
The group thanks Joana Espricigo Conte-Spilari, Camila Marques Bertolucci, Alef Rafael Severino, Diana AC Kluck, Raysa Rosseto Rodrigues, Vanessa Kukla and Regielly Cognialli for the technical support. This research was not funded by any specific grant from funding agencies in the public, commercial or not-for-profit sectors.