The coronavirus disease 2019 (COVID-19) caused by the SARS-CoV-2 virus is a global pandemic with more than 100 million recorded cases to the present date1. By mechanisms that are still poorly understood, this infection is associated with several hematological complications. Initial data show that such events are the result of the state of hypercoagulability caused by the immune response to infection by SARS-CoV-2.2
Autoimmune diseases have been reported in association with this pandemic disease, among which we find in the literature few reports of COVID-19 associated with thrombotic thrombocytopenic purpura (TTP).3–5
TTP is characterized by systemic microvascular thrombosis with platelet aggregation. It typically presents with hemolytic anemia, thrombocytopenia, renal dysfunction, fever and neurological involvement, to varying degrees. This condition is associated with deficiency of the enzyme responsible for the cleavage of the von Willebrand factor, disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13). The reduction in the ADAMTS13 activity (< 10%), together with the presence of inhibitory anti-ADAMTS13 autoantibodies, suggests acquired TTP.6 Infections are often associated as triggering factors for the initial manifestation of autoimmune thrombotic purpura, as well as with exacerbations of the disease7. Our case reports a rare association of acquired TTP in a pediatric patient with recent SARS-CoV-2 infection.
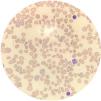
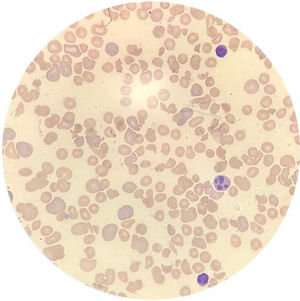
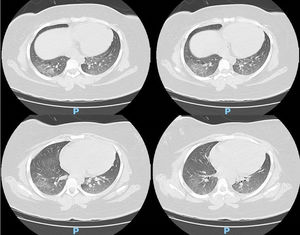
Case reportA 14-year-old female patient, who had a past medical history of depression was transferred to our hospital in August 2020 due to headache and altered level of consciousness. The patient had past symptoms of fever (body temperature 38 °C), cough, dyspnea, fatigue and malaise for approximately 11 days, that resolved spontaneously about twenty days before hospitalization and during the same period her parents had similar symptoms, but received no medical care. The initial laboratory screening (Table 1), showed anemia, with the presence of schistocytes in a peripheral blood smear (Figure 1), thrombocytopenia and alteration of hemolysis markers. The direct Coombs test was negative. The coagulogram and serum fibrinogen were within the normal range. Routine laboratory tests from five months earlier had no remarkable findings. The cranial computed tomography was unremarkable. Due to recent respiratory symptoms, a computed tomography (CT) scan of the chest was performed, which showed peripheral and bilateral ground-glass opacities suggestive of viral pneumonia (Figure 2). The reverse-transcriptase polymerase chain reaction (RT-PCR) test for COVID-19 was performed and it presented a negative result. The serological test for COVID-19 showed a positive IgG fraction, proving previous exposure to SARS-CoV-2. The diagnosis of acquired thrombotic thrombocytopenic purpura was confirmed by the low activity of the ADAMTS13 at < 5% (reference > 70%), with a positive test for anti-ADAMTS13 inhibitors at 4.6 (reference < 0.4). Additional laboratory tests, such as the antinuclear antibody, anti-double stranded DNA (anti-dsDNA), complement fractions, anti-Ro, anti-La, serologies for viral hepatitis and human immunodeficiency virus (HIV), were performed as a screening for conditions associated with TTP and resulted negative. A central venous catheter was introduced and plasma exchange therapy (PEX), along with the systemic corticosteroid methylprednisolone at 1 mg per kilogram, was initiated. During treatment, the patient clinical status deteriorated due to venous catheter-related infection, which was treated with blood culture-guided vancomycin for methicillin-resistant Staphylococcus aureus (MRSA). A total of 25 daily PEX sessions were performed during the treatment, the average volume exchanged being 5300 mL per session, corresponding to approximately 1.0 times the patient volemia. She evolved with the recovery of the red and platelet series and hospital discharge on the 38th day of hospitalization for outpatient follow-up. The serological test for SARS-CoV-2 was repeated at the discharge and it also showed a positive IgG fraction.
Laboratory evolution.
MCV: mean corpuscular volume; LDH: lactate dehydrogenase; GOT: glutamic oxaloacetic transaminase; GPT: glutamic pyruvic transaminase; aPTT: activated partial thromboplastin time; INR: international normalized ratio; CRP: c-reactive protein; N/A: not available.
TTP in the pediatric age group is a rare disease, with an incidence of approximately 1 case per 1 million children, which predominates in females with a median age of 13 years.8 The case presented here suggests an unusual association, given that there are few cases in the literature to the present date, suggesting a relationship between COVID-19 and this hematological condition, being that our PubMed research only found data on the adult population.
Infections in general are among the main factors that trigger this condition through endothelial activation by nucleosomes that are derived from the cellular content released by neutrophils in response to the infectious condition.7 Capecchi et al. suggest that endothelial activation may happen late in the coronavirus infection.5 The reported case resembles the fact that the RT-PCR for COVID-19 was negative and had serology proving previous infection, symptomatology and a compatible chest CT image. Albiol et al. describe a 57-year-old patient with acquired microangiopathic thrombosis secondary to viral infection by the coronavirus with a false negative RT-PCR for COVID-19,3 corroborating the hypothesis that this hematological condition may be an uncommon complication triggered by the pandemic viral infection by SARS-CoV-2.
Although the prognosis of the disease has improved considerably with the use of PEX, it remains a serious condition with a mortality rate of approximately 10%, despite the therapy.8,9 The patient in the case described was treated with daily sessions of plasma exchange and adjuvant systemic corticosteroids, as suggested in the literature, and despite the infectious complication, she recovered from the acute condition and received hospital discharge for follow-up.6
Studies show that TTP has a high risk of relapse, especially during the first year of the disease and in patients with positive anti-ADAMTS13 inhibitors. The patient in the reported case can be considered at high risk for relapses, which are associated with greater morbidity and mortality.10 Evidence shows that rituximab can be used to prevent relapse of the disease, especially in patients at high risk, as the report presented. However, it is not immediately available at our service, being used only in refractory cases. Other promising therapies described, such as n-acetylcysteine and recombinant ADAMTS13, were not used due to the good response to the initial treatment.9
Other conditions previously described as triggering factors for this hematological disease were investigated and discarded, such as systemic lupus erythematosus, systemic sclerosis, neoplasia and other infectious diseases. No other more probable cause for the microangiopathic thrombotic disease was found.
The serological test (Advagen Biotech Ltda.) using the immunochromatographic method with 95.2% sensitivity for IgG and 100% specificity for IgG and a general precision of ∼92% was performed twice at patient admission and discharge and had both positive results for the IgG fraction. This test is approved by the Brazilian Health Regulatory Agency.11
Elefante et al. suggest in their report that suspicion of COVID-19 infection should not only be based on isolated tests, especially in patients with systemic autoimmune conditions.12 There is currently no information about TTP leading to false-positive SARS-CoV-2 serological tests.
The main limitation of this report is that there is not enough evidence in the literature to conclude that the acquired thrombotic thrombocytopenic purpura was caused by the immune response to SARS-CoV-2, which may be a case of chance due to the high prevalence of COVID-19.
This report suggests that this pandemic infectious disease is one of several possible causes of acquired TTP. The immunological deregulation caused by late SARS-CoV-2 infection could have precipitated this hematological disorder. We believe that further studies should be published to confirm the existence of the causal factor between these two conditions. TTP should be suspected in the presence of characteristic signs and symptoms, such as thrombocytopenia, neurological symptoms and signs of hemolytic anemia with schistocytes in the peripheral blood smear, and treated promptly to reduce the risk of mortality. COVID-19 should be part of the investigation at the onset of TTP with no obvious cause, especially in the presence of respiratory symptoms and a compatible chest image.