This study aimed to determine the clinical outcomes and risk factors affecting mortality in patients with COVID-19 following hematological malignancy (HM).
MethodsPatients diagnosed with HM and hospitalized for COVID-19 were included in this retrospective study. The age, demographic and clinical characteristics, prognosis and treatment of surviving and non-surviving patients were compared.
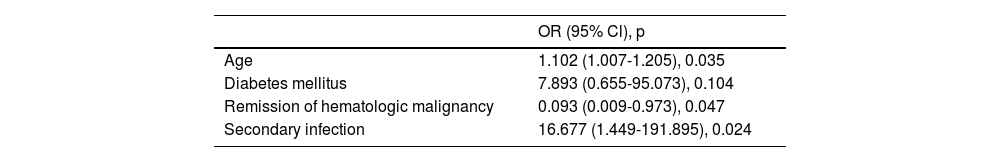
ResultsA total of 49 patients were included in this study, 17 (34.6%) of whom died within 28 days of being diagnosed with COVID-19. Older age (p = 0.001), diabetes (p = 0.001), chronic obstructive pulmonary disease (p = 0.002), secondary infection (p < 0.001) and secondary bacterial infection (p = 0.005) were statistically significantly higher in non-survivors. The remission status of HM was higher in surviving patients (p < 0.001). In multivariate regression analysis, age (OR: 1.102, p = 0.035) and secondary infection (OR: 16.677, p = 0.024) were risk factors increasing mortality, the remission status of HM (OR: 0.093, p = 0.047) was a protective factor from mortality.
ConclusionThe older age, the remission status of HM and secondary infection due to COVID-19 were determined as prognostic factors predicting mortality in HM patients with following COVID-19.
Hematological malignancies (HMs) are one of the leading causes of mortality and morbidity worldwide.1 The patient with HM usually has long-term immunodeficiency due to the malignancy, anti-cancer treatments or procedures, such as hematopoietic stem cell transplantation (HSCT).2 These people are very concerned about the high risk of morbidity and mortality from COVID-19. According to the literature, the overall mortality associated with COVID-19 among hematologic patients ranges from 32% to 40%.3
Recent studies have demonstrated that COVID-19 has a two-phase illness process. The classic upper respiratory tract infection symptoms are observed during the first phase. If the disease does not improve, it progresses to the second phase and a 'cytokine storm' develops, in which increased cytokine levels characterize an enhanced immune response. There are dyspnea and hypoxia, with infiltrates in the lung.4-6
It was determined that activated and depleted CD8+ T cells were associated with the severity of the disease in COVID-19.7 In the early stages of the pandemic, the cytokine storm seen in the course of COVID-19 was identified. It was thought that the existing immunodeficiency in patients with HM would not allow for the development of a cytokine storm and would limit the progression to severe/critical COVID-19. However, real-life data indicate that morbidity and mortality rates are variable. The subtype and activation status of HM, age and comorbidities were identified as factors influencing mortality.8,9
This study aimed to determine the factors that affect the clinical development and prognosis of HM patients treated for COVID-19.
MethodsThis retrospective study was conducted in a tertiary hospital with a total of 1,600 beds and a hematology unit with a capacity for 24 patients.
Patients and definitionsPatients with a diagnosis of HM and COVID-19 who were hospitalized in the Kayseri City Training and Research Hospital between April 2020 and October 2021 were included in this retrospective study. Demographic characteristics, HM diagnoses and clinical conditions (new diagnosis, remission and recurrence) of the patients and the clinical course and treatments for COVID-19 were recorded. During the COVID-19 process, 28-day mortality cases were evaluated. The patients were divided into two groups: survivors and non-survivors and the factors predicting mortality were determined.
Patients with PCR-positive SARS-CoV-2 were diagnosed with COVID-19. According to the clinical severity classification of COVID-19, patients with fever and cough, but with normal O2 saturation and no change in X-rays and/or CT scans, were classified as mild COVID-19; patients with ground glass opacities, lung consolidation and O2 saturation < 94%, as moderate COVID-19, and; patients with PaO2/FiO2 < 300 mmHg and respiratory frequency > 30 breaths/min., as severe/critical COVID-19.6
Diagnosis and treatment strategies for HM were determined according to the National Comprehensive Cancer Network (NCCN).10 The COVID-19 treatment was carried out in accordance with the COVID-19 treatment management guidelines of the Ministry of Health. 11 Favipiravir is given as an antiviral drug to patients who have received treatment for COVID-19. The IL-6 inhibitor tocilizumab, steroids and high-dose steroids were used in accordance with the guidelines for patients with increased inflammatory markers. 12
Secondary bacterial infections were defined as bacterial infections occurring during the COVID-19 disease course or hospital stay (> 48 - 72 h after hospitalization).13 Secondary fungal infections were defined according to the CDC.14
Statistical analysisThe statistical analysis was performed using the SPSS 22.0 (IBM Corp., Armonk, NY, USA) package program. The Shapiro-Wilk test was performed to check the normality assumption of the data. Categorical variables were expressed as numbers and percentages and Chi-square or Fisher's Exact Test analysis was used for comparisons. Variables with a p-value ≤ 0.05 were included in the multivariate logistic regression analysis. A p-value ≤ 0.05 was considered statistically significant in all analyses.
Ethical approvalThis study was approved by the Kayseri City Hospital, Clinical Research Ethics Committee (Date: 24.12.2020 and Approval Number: 251) and was conducted in accordance with the Principles of the Declaration of Helsinki. As it was performed retrospectively by the file scanning method, an 'informed voluntary consent form' was not obtained from the patients.
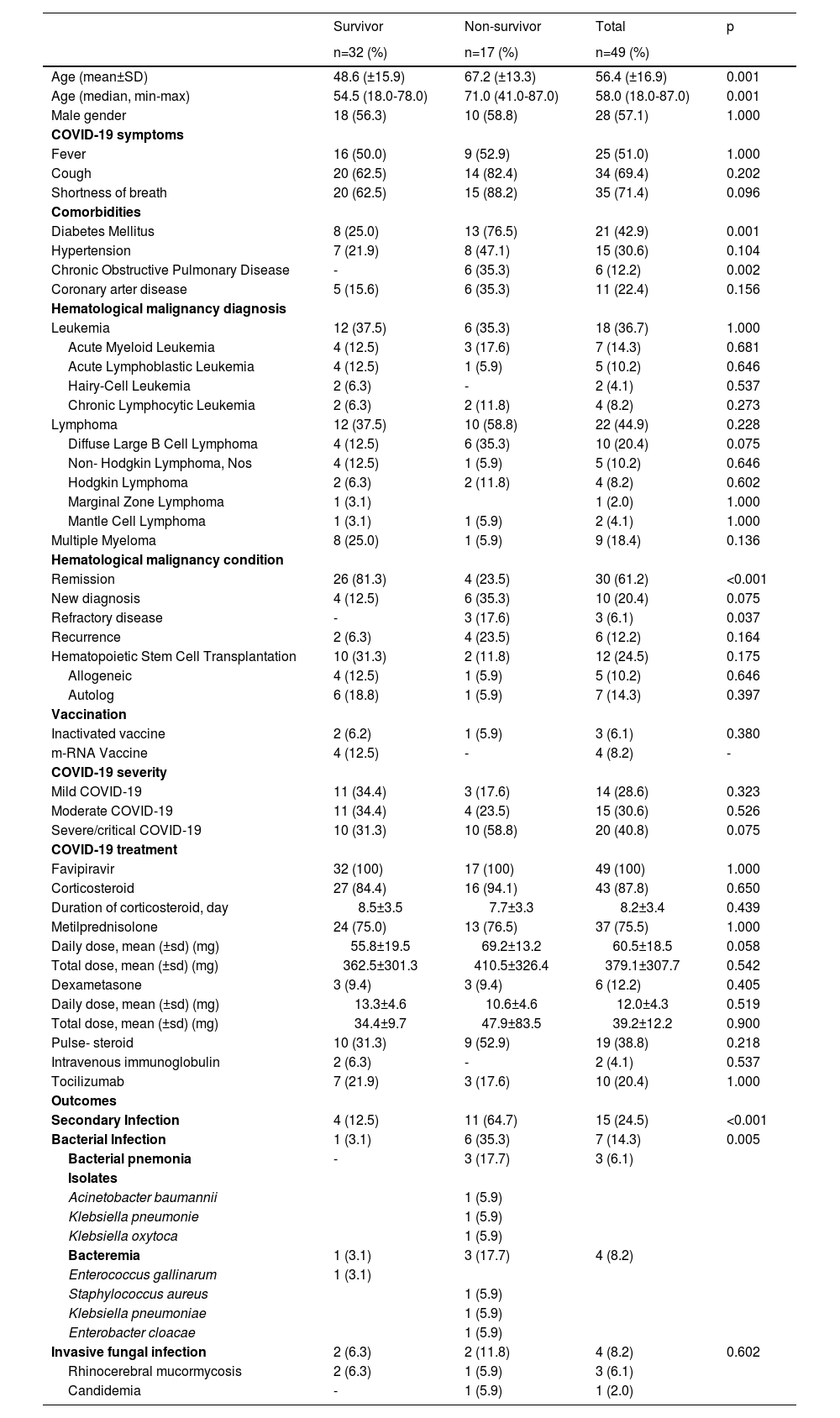
ResultsA total of 49 patients were included in the study. The clinical and demographic data of the patients are presented in Table 1. Twenty-eight (57.1%) patients were male and the mean age was 57.90 ± 16.43 years. Of the patients, 17 (34.6%) died within 28 days of being diagnosed with COVID-19 and 32 (65.3%) of them recovered. The mean age of the non-survivor group was 67.23 (± 13.33) and of the survivor group, 48.68 (± 15.95), this difference is statistically significant (p < 0.001). At the time of admission, complaints of fever, cough and shortness of breath were similar in both groups (p = 1.000, p = 0.20,2 and p = 0.096, respectively). The prevalence of diabetes was 76.5% in the survivor group and 25.0% in the non-survivor group and this difference was statistically significant (p = 0.001). When the frequency of diagnosis of HM was evaluated, it was observed that 22 (44.9%) patients were followed up for lymphoma, 18 (36.7%) patients, for leukemia, and 9 (18.4%) patients, for multiple myeloma. No statistically significant difference was found between the survivor and non-survivor groups in the type of HM (p > 0.05). The HM was evaluated for disease activation status and 81.3% of the survivors and 23.5% of the non-survivors were in remission (p < 0.001). A total of 12 (24.5%) patients were HSCT recipients. Allogeneic HSCT was performed in three patients (9.1%) and autologous SCT was performed in six patients (18.8%). The frequency of patients receiving HSCT and the frequency of performing allogeneic and autologous HSCT were found to be statistically similar between survivor and non-survivor patients (p = 0.175, p = 0.646, and p = 0.397, respectively).
Demographic and clinical characteristics of patients
According to the clinical severity of COVID-19, a total of 14 (28.6%) patients were mild, 15 (30.6%) patients were moderate, and 15 (40.8%) patients severe/critical COVID-19. There was no difference between the two groups in the classification according to the clinical severity of COVID-19 (p = 0.323, p = 0.526 and p = 0.075, respectively).
The rate of corticosteroid and tocilizumab used for the treatment of COVID-19 was similar in the survivor and non-survivor groups (p = 0.650 and p = 1.000, respectively).
In a total of 15 patients (24.5%), secondary infection developed while being followed up for COVID-19. Seven (14.3%) of these were bacterial infections, while four (8.2%) were invasive fungal infections. Secondary bacterial infection was seen in 6 (35.3%) in non-survivors and in one (3.1%) patient from the survivors (p = 0.005). Bacterial pneumonia was observed in three patients and bacteremia was observed in four patients as the secondary bacterial infection. An Acinetobacter baumannii strain causing bacterial pneumonia and a Klebsiella pneumoniae strain had carbapenem resistance. In addition, the Klebsiella pneumoniae strain, isolated as a bacteremia agent, was resistant to carbapenem and the Staphylococcus aureus strain was resistant to methicillin.
The frequency of invasive fungal infections was similar in both groups (11.8% vs. 12.5%, p = 1.000). Rhinocerebral mucormycosis was seen in three patients. The only patient with candidemia died.
In multivariate regression analysis (Table 2), older age (OR: 1.150, p = 0.024) and secondary infection (OR: 16.677, p = 0.024) were evaluated as factors predicting mortality in patients with HM. Being in remission of the HM was found to be a factor reducing mortality (OR: 0.093, p = 0.047).
DiscussionThis study evaluated the clinical features and risk factors affecting the mortality of patients with HM diagnosed and followed up for COVID-19. Older age and secondary infection have been identified as risk factors that increase mortality. Diabetes was observed more frequently in patients who died, but it was not considered a risk factor. The remission of HM was found more in surviving patients and was evaluated as a factor in reducing the risk of mortality.
During the COVID-19 pandemic, patients with HM are a potentially high-risk population due to immunosuppressive therapies.15 The immune dysfunction of patients due to HM also affects the course of COVID-19 and there are important risk factors affecting mortality in these patients. It has been reported that older age increases mortality 1 to 13 times in COVID-19 patients without malignancy.16,17 In addition, infectious complications in patients with HM increase with age and older age is associated with a poor prognosis.18 There are few studies on COVID-19 patients with HM and older age has been reported as a risk factor that increases mortality and morbidity.3,9 According to the results of our study, it was determined that the increased mean age increased mortality 1.1 times.
Diabetes and impaired glucose tolerance have been associated with poor prognosis in COVID-19 disease.19 In a meta-analysis of 30 studies and 6,452 patients, it was stated that the presence of DM led to a two-fold increased mortality risk and severity of COVID-19.20 According to our study results, diabetes was found with a higher frequency in patients who died.
The remission of HM is known to be a good prognostic factor, especially in infectious complications.21 By controlling the malignancy, it is possible to regain the immune system functions. In a study conducted in Spain, in which 367 patients with HM who had COVID-19 were evaluated, uncontrolled HM was associated with an increased 45-day mortality rate.3 In another study, conducted in the early period of the COVID-19 pandemic and which evaluated 34 patients with HM, it was found that patients in remission without active malignancy had a better survival rate. In our study, the remission of HM was similarly found to be a factor in reducing mortality due to COVID-19.
Bacterial/fungal secondary infections contribute to the increased morbidity and mortality of viral respiratory infections.22 It has been reported that the frequency of infections caused by resistant bacteria has increased due to inappropriate antibiotic use and extended hospitalization in the intensive care unit.23 In addition, it has been reported that respiratory tract damage caused by SARS-CoV-2 and alveolar damage caused by the cytokine storm increase the risk of invasive fungal infection.24
ConclusionOlder age, remission of HM and secondary infection were determined as prognostic factors predicting mortality in HM patients followed up with COVID-19.
There are some limitations in this study. This study was retrospectively performed at a single center. The details of chemotherapy regimens could not be fully reached. In addition, the small number of groups prevented sub-analysis between different hematological malignancies and thus, prospective studies with large patient groups are needed.
Ethics statementThe clinical research ethics committee of Kayseri City Hospital approved this research (Date: 12/24/2020 Number: 251).