Little attention is given to thrombosis associated with pediatric acute promyelocytic leukemia (APL). This study describes the thrombotic and hemorrhagic manifestations of APL in pediatric patients and evaluates their hemostasis, based on coagulation tests.
MethodsInclusion criteria were age 0–18 years and APL diagnosis between April 2005 and November 2017. Patients who had received blood transfusion prior to coagulation tests were excluded. Baseline coagulation tests, hematologic counts, and hemorrhagic/thrombotic manifestations were evaluated.
ResultsMedian age was 10.7 years (1–15 years). The initial coagulation tests revealed a median Hgb of 8.3 g/dL (4.7–12.9 g/dL), median leucocyte count of 10.9 × 10⁹/L (1.1–95.8 × 10⁹/L), median platelet count of 31.8 × 10⁹/L (2.0–109.0 × 10⁹/L), median activated partial thromboplastin time (aPTT) of 31.7 s (23.0–50.4 s), median aPTT ratio of 1.0 (0.78–1.6), median thromboplastin time (PT) of 17.5 s (13.8–27.7 s), median PT activity of 62% (25–95 %), and median fibrinogen of 157.7 mg/dL (60.0–281.0 mg/dL). Three patients (13%) had thrombosis. At diagnosis, 21 patients (91.3%) had bruising, one patient (4.3%) had splenic vein and artery thrombosis and one patient (4.3%) presented without thrombohemorrhagic manifestations. During treatment, two patients (8.6%) had thrombosis.
ConclusionKnowledge of thrombosis in pediatric APL is important to determine its risk factors and the best way to treat and prevent this complication.
Acute promyelocytic leukemia (APL) accounts for 10–15 % of pediatric acute myeloid leukemia (AML) and is characterized by a recurrent translocation between chromosomes 15 and 17 and a complex coagulopathy.1 There are two main mechanisms involved in the hemorrhagic and thrombotic events of APL, namely disseminated intravascular coagulation (DIC) induced by the overexpression of the tissue factor (TF) in the APL blast and primary hyperfibrinolysis induced by the expression of annexin II.2 The APL DIC is different from the variant form secondary to sepsis. In general, the levels of natural anticoagulants, such as protein C, protein S, and antithrombin III are normal in APL, whereas in DIC secondary to sepsis, these proteins are reduced. In addition, hyperfibrinolysis plays a key role in APL hemorrhage physiopathology.3
At initial presentation, the majority of APL patients manifest with bleeding in the form of bruises, petechiae, epistaxis, and menorrhagia.2 Even with the advent of all-trans-retinoic acid (ATRA) and the improvements in transfusion support, hemorrhagic complications in the central nervous system (CNS) and lungs remain the major cause of early death in 5–9 % of patients in clinical trials.4 As in adults, a high leukocyte count (>10.0 × 10/L) is considered a risk factor for early death secondary to hemorrhage in pediatric patients.5
While the hemorrhagic complications of APL have been extensively studied, little is known about its thrombotic manifestations, especially in pediatric patients. A few hypotheses have been proposed to explain the increased tendency for thrombosis in APL, including a manifestation of DIC, the use of ATRA, the combined use of ATRA and antifibrinolytic agents and a manifestation of the differentiation syndrome6. Following the introduction of ATRA as the cornerstone of treatment for APL, the incidence of thrombosis in patients with APL has ranged between 5–19%.7–9 However, the morbidity and mortality associated with thrombotic complications in APL patients are unknown. Because APL is a rare disease, especially in children and adolescents, data on hemostasis and thrombosis in APL are mainly available from studies with adults.5
ObjectiveThe purpose of the study was to describe the demographic characteristics and hemorrhagic and thrombotic manifestations of a cohort of pediatric patients with APL and to evaluate their hemostasis condition at diagnosis, using basic coagulation tests.
Material and methodsThis descriptive and retrospective study was approved by the Research Ethics Committee of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP), São Paulo, Brazil. Medical records of patients from the HCFMUSP Children and Adolescent’s Institute with a diagnosis of APL with a minimum of 1 year of follow-up after the end of treatment were evaluated. Inclusion criteria were: age 0–18 years, APL diagnosis between April 2005 and November 2017 and genetic confirmation of the APL diagnosis by detection of PML-RARα fusion transcript by reverse-transcription PCR or demonstration of the t(15;17) translocation by karyotyping or fluorescence in situ hybridization (FISH). Patients who had received blood components before referral to our center (platelet concentrate, fresh frozen plasma, or cryoprecipitate) before the coagulation tests were excluded. Clinical and laboratory data at initial presentation, including age, gender, hemoglobin (Hgb), white blood cell count (WBC), platelet number, fibrinogen level, activated partial thromboplastin time (aPTT)/aPTT ratio, prothrombin time (PT)/prothrombin activity, hemorrhagic/thrombotic manifestations and outcome during induction therapy, were retrieved from the medical records. Unfortunately, there was no record of laboratory tests immediately before thrombotic and hemorrhagic events for most patients.
ResultsOf the 25 patients initially evaluated, two were excluded because they received transfusion before being admitted to our service. All patients were treated with a modified form of the automatic interpretation for diagnostic assistance (AIDA) protocol and received ATRA (25–45 mg/m² until remission) and idarrubicin (days 2,4,6,8) at induction. The median age was 10.7 years (range: 1.0–15.0 years) and 15 patients (65.2 %) were girls. Peripheral blood counts showed a median Hgb level of 8.3 g/dL (range: 4.7–12.9 g/dL), median WBC of 10.9 × 10⁹/L (range: 1.1–95.8 × 10⁹/L) and median platelet count of 31.8 × 10⁹/L (range: 2.0–109.0 × 10⁹/L).
The initial coagulation tests revealed a median aPTT of 31.7 s (range: 23.0–50.4 s), median aPTT ratio of 1.0 (range: 0.78–1.6), median PT of 17.5 s (range: 13.8–27.7 s), median PT activity of 62% (range: 25–95 %) and a median fibrinogen level of 157.7 mg/dL (range: 60.0–281.0 mg/dL). Fibrinogen below 200.0 mg/dL was present in 60% of the patients, of whom 43% had a fibrinogen level below the minimal hemostatic level of 100.0 mg/dL. The PT and aPTT were prolonged in 69% and 13% of the patients, respectively.
At initial presentation, 21 patients (91.3%) had mucocutaneous bleeding, one patient (4.3%) had no bleeding manifestations, but presented with major splenic vein and artery thrombosis, and one patient (4.3%) presented with isolated pancytopenia, but no thrombohemorrhagic manifestations. During the induction phase, 8.6% of the patients (2/23) developed thrombotic manifestations (one femoral vein thrombosis and one cephalic thrombophlebitis). There were two deaths, one secondary to a CNS hemorrhage during the induction phase and one secondary to sepsis during the maintenance phase in a patient in clinical and molecular remission.
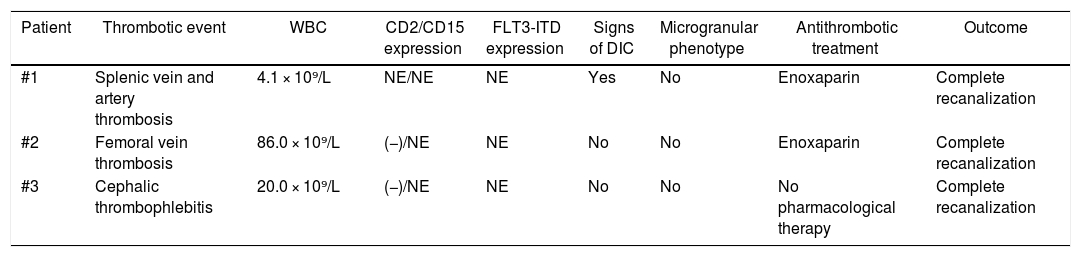
Three patients (13%) experienced thrombotic events, one of which having surprisingly occurred at initial presentation. The patient with splenic vein and artery thrombosis had signs of DIC, but no leukocytosis (WBC: 4.1 × 10⁹/L). The initial immunophenotyping was inconclusive, so we were unable to determine the expression of CD2 and CD15 and FLT3-ITD was not evaluated in this patient. The patient with femoral vein thrombosis had a WBC count of 86.0 × 10⁹/L, but only exhibited prolonged PT. No CD2 expression was detected in this patient and CD15 and FTL3-ITD expression were not evaluated. The patient with cephalic vein thrombophlebitis had a WBC count of 20.0 × 10⁹/L, showed no changes in the coagulation tests and was negative for CD2 and FLT3-ITD expression, but CD15 expression was not evaluated. None of the patients with thrombotic events had a microgranular phenotype. The patient with thrombophlebitis did not receive antithrombotic treatment, whereas the other two were treated with enoxaparin (1 mg/kg/dose, twice a day) with complete vein recanalization and no hemorrhagic complications. The one with splenic vein and artery thrombosis received anticoagulation for 6 months and the one with femoral vein thrombosis received anticoagulation for 3 months. The information on thrombotic events and associated risk factors is summarized in Table 1.
Thrombotic events and risk factors.
| Patient | Thrombotic event | WBC | CD2/CD15 expression | FLT3-ITD expression | Signs of DIC | Microgranular phenotype | Antithrombotic treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| #1 | Splenic vein and artery thrombosis | 4.1 × 10⁹/L | NE/NE | NE | Yes | No | Enoxaparin | Complete recanalization |
| #2 | Femoral vein thrombosis | 86.0 × 10⁹/L | (−)/NE | NE | No | No | Enoxaparin | Complete recanalization |
| #3 | Cephalic thrombophlebitis | 20.0 × 10⁹/L | (−)/NE | NE | No | No | No pharmacological therapy | Complete recanalization |
NE: not evaluated.
In our series, almost all the patients had thrombohemorrhagic manifestations as the initial presentation of APL. A large series of APL patients revealed that roughly 89% of subjects had hemorrhagic manifestations, mainly mucocutaneous bleeding.2 Prolonged TP and thrombocytopenia are the more commonly found in hemostasis laboratory findings in APL patients and this was also the case in our study. There was one early death secondary to hemorrhage, an early death rate (4.3%), similar to rates of large clinical trials.4
Thrombosis is a less recognized, underrated and sometimes life-threatening manifestation of APL that is overshadowed by the more common hemorrhagic complications. A substantial incidence of thrombosis has been found in large series of patients with APL, especially after the introduction of ATRA.6 A number of risk factors have been implicated in the occurrence of APL thrombosis, including, among others, leukocytosis (WBC count: >30 × 10⁹/L), signs of DIC, presence of FLT3-ITD, expression of the bcr3 PML-RARA isoform and CD2 and CD15 expression.10 The PETHEMA group also considered the microgranular subtype and low fibrinogen levels as risk factors.6 In a retrospective analysis, Bai et al. (2019) showed that in the group of patients with a WBC count >10.0 × 10⁹/L, those with thrombotic manifestations had lower FDP/FIB (fibrinogen degradation products/fibrinogen) and D-Dimer/FIB ratios than those with hemorrhagic manifestations.11
ATRA is believed to induce a state of hypercoagulability after the rapid correction of the fibrinolysis syndrome and it also induces the expression of some adhesion molecule, such as Very Late Antigen-4 (VLA-4) and Lymphocyte Function-Associated 1 (LFA1).10 Before the advent of ATRA, the incidence of APL-associated thrombosis was estimated to be 2%, but it reaches 19% with current protocols.6 Anthracyclines are also involved in the tendency for thrombosis in APL because of increased TF activity and release of TF-bearing microparticles, induced by apoptotic leukemia cells.6 In a retrospective analysis of APL patients older than 16 years of age, Silva et al. reported that 26% of patients had thrombosis, of whom all had received ATRA before the thrombotic events.12
APL thrombosis may occur at any time during treatment; it is estimated that 40.4% of all APL-associated thrombosis episodes occur before treatment, 43.6% during induction, and approximately 14% after induction.6 There are case reports of thrombotic complications in pediatric patients associated with high morbidity and mortality, including sinus vein thrombosis,13,14 inferior vena cava thrombosis with acute Budd-Chiari syndrome15 and splenic, renal, and intestinal artery thrombosis16.
Our study has some limitations, such as being a retrospective analysis, the inclusion of only one center, the lack of laboratory data (e.g., D-dimer in most patients) and immunophenotyping parameters (CD2 and CD15) and few patients had FLT3-ITD information. The small number of patients analyzed and the few with thrombosis does not allows us to make any conclusions, but alert us about the existence of this complication in pediatric patients and its different clinical presentations. The two deaths included in our cohort, especially the one during the induction phase, are presented as a bias, since these patients could not progress with thrombosis during the disease treatment. The exclusion of patients that received blood components before admission to our service could also constitute a bias, since these patients could be considered a more severe form of the disease and, therefore, at greater risk for thrombosis. Finally, because of the study design, we may have lost some of the patient records. Our study focused on reporting APL-associated thrombotic complications in pediatric patients, but also their hemorrhagic manifestations and laboratory hemostasis data. Some of the risk factors for thrombosis described in adult APL patients were also present in the children and adolescents in our study. The anticoagulation treatment seems to be safe and should not be delayed if the patient has no contraindications. Because of the clinical similarity to retinoic acid syndrome, thrombosis should not be overlooked as an important differential diagnosis.
ConclusionHemorrhagic complications remain the major cause of death in APL, but thrombotic manifestations are also a relevant part of APL coagulopathy. The procoagulant tendency of this subtype of AML is related, not only to its own physiopathology features, but also to its treatment. Pediatric hematology oncologists need to be aware of this complication and its treatment should not be delayed because of the bleeding tendency in APL patients, its most feared complication. Unexplained thrombosis in an otherwise healthy child should be a warning sign for APL. More studies in pediatric populations will help to establish the specific risk factors and the best way to treat and prevent thrombosis in APL.
Conflict of interest statementThe authors whose names are listed below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patentlicensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations knowledge or beliefs) in the subject matter or materials discussed in this manuscript.