To study systemic lupus erythematosus in a Brazilian population using the American College of Rheumatology hematological classification criteria and report associations of the disease with serological and clinical profiles.
MethodsThis is a retrospective study of 460 systemic lupus erythematosus patients followed in a single rheumatologic center during the last 10 years. Hematological manifestations considered for this study were hemolysis, leukopenia, lymphocytopenia and thrombocytopenia.
ResultsThe cumulative prevalences of leukopenia, thrombocytopenia, lymphocytopenia and hemolytic anemia were 29.8%, 21.08%, 17.7% and 8.4%, respectively. A higher percentage of patients with hemolysis had anticardiolipin IgM (p-value=0.002). Those with leukopenia had more lymphopenia (p-value=0.02), psychosis (p-value=0.01), thrombocytopenia (p-value <0.0001) and anti-double stranded DNA antibodies (p-value=0.03). Patients with lymphopenia had more leukopenia (OR=1.8; 95% CI=1.01–3.29) and lupus anticoagulant antibodies (OR=2.2; 95% CI=1.16–4.39) and those with thrombocytopenia had more leukopenia (OR=3.1; 95% CI=1.82–5.44) and antiphospholipid syndrome (OR=3.1; 95% CI=1.28–7.87).
ConclusionThe most common hematological finding was leukopenia and the least common was hemolysis. Associations of low platelet count and hemolysis were found with antiphospholipid syndrome and anticardiolipin IgM positivity, respectively. Leukopenia and lymphocytopenia are correlated and leukopenia is more common in systemic lupus erythematosus patients with psychosis, thrombocytopenia and anti-double stranded DNA.
Systemic lupus erythematosus (SLE), a systemic autoimmune disease most common in young females, has a very heterogeneous clinical profile.1 The genetic background of patients affects not only the prevalence of SLE but also the phenotype.2 Accordingly ethnic features favor the appearance of autoantibodies and clinical clusters that define the subtypes of the disease.3,4 These aspects highlight the need to know lupus clusters as this awareness allows the clinician to predict a future manifestation from one already present. It also highlights the need for local knowledge of disease behavior, particularly in a population such as the Brazilian which is highly mixed from the ethnic point of view.
The classical hematological manifestations in SLE are hemolytic anemia, leukopenia, and thrombocytopenia; these manifestations are part of the 1997 revised American College Classification Criteria for SLE5 as well as the new 2012 Systemic Lupus International Collaborating Clinics Classification Criteria.6
According to previous works, thrombocytopenia has a prevalence in the lupus population ranging from 7 to 30%.7–9 Although thrombocytopenia is not directly associated with end organ damage, it defines a subgroup of patients with higher morbidity and consequently has important prognostic implications.10
Leukopenia is a typical feature of SLE and may occur as a result of lymphopenia, neutropenia or both.11 Neutropenia, which may be mediated by anti-neutrophil antibodies, is common, with a prevalence in the order of 47%.11,12 The prevalence of lymphopenia is variable, ranging from 20 to 81% and correlates with disease activity.12,13 Both T and B lymphocytes are reduced while natural killer (NK) cells are elevated.11,14 Although there are numerous reports of lymphocytotoxic antibodies,11,15 their significance in this context remains uncertain. Reduced surface expression of complement regulatory proteins such as CD55 and CD59 has also been implicated in the pathogenesis of lupus lymphopenia, as this deficiency will make cells susceptible to complement-mediated lysis.11,16
Autoimmune hemolytic anemia (AIHA) is described in 7–15% of lupus patients and may occur together with immune thrombocytopenia in the Evans syndrome.17,18 It is associated with the presence of warm (predominantly) and cold anti-red blood cell autoantibodies.17
The aim of the current study was to assess the prevalence of hematological manifestations in a cohort of Brazilian lupus patients as well as its associations with clinical and autoantibody profiles.
MethodsThis is a retrospective study, approved by the local Research Ethics Committee. The charts of 460 SLE patients seen over the last 10 years in a single tertiary center were reviewed. To be included in this study, patients had to comply with at least four of the 1997 revised American College of Rheumatology classification criteria for SLE.5 Patients diagnosed before the age of 16 years and those with incomplete records were excluded. Data on demographic, clinical and serological profile were obtained. The definition of all clinical findings followed those of the ACR classification criteria.5 The criteria were cumulatively considered when the patient had no known infections. According to these criteria, hematological manifestations were defined as the presence of hemolytic anemia, leukopenia defined as less than 4×103 cells/mL on at least two occasions, lymphopenia defined as less than 1.5×103 cells/mL on at least two occasions and thrombocytopenia defined as less than 100×103 cells/mL in the absence of an offending drug.5 Antiphospholipid syndrome (APS) was diagnosed according to the 2006 modified APS criteria.19 The complete cell count was performed using an automated analyzer (XE2100D, Sysmex) and the white cell differential count was performed manually using Giemsa stain.
Statistical analysisAll obtained data were collected as frequencies in contingency tables. The Kolmogorov–Smirnov test was used to study data distribution. Groups of patients with one hematological manifestation (hemolytic anemia, leukopenia or thrombocytopenia) were compared with those without this particular manifestation in respect to other clinical manifestations and their autoantibody profile. Central tendency was expressed as median and interquartile range (IQR) when numeric data were nonparametric and mean and standard deviation (SD) when parametric. Association studies were performed by Fisher's exact and chi-square tests for nominal data and with Mann–Whitney and unpaired t-test for numerical data. All variables that had significance with a p-value <0.1 in univariate analysis, were further studied using logistic regression to assess independency. Statistical analyses were made using the Medcalc software version 10.0, and significance was set for an alpha error of 5%.
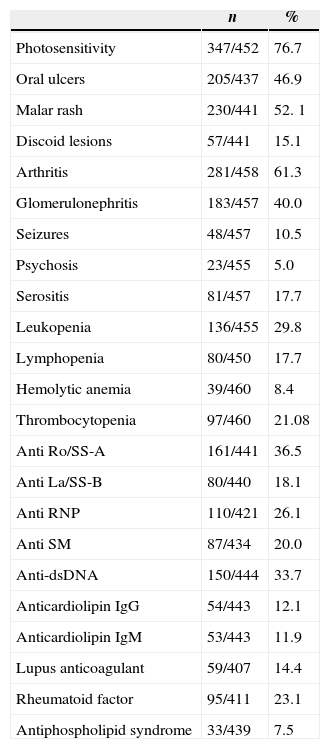
ResultsAnalysis of the sampleThe sample was comprised of 93.5% females and 6.5% males with ages ranging from 16 to 88 years and median disease duration of 8 years. The clinic and serological profiles are listed in Table 1.
Clinical and serological profile of lupus patients.
| n | % | |
|---|---|---|
| Photosensitivity | 347/452 | 76.7 |
| Oral ulcers | 205/437 | 46.9 |
| Malar rash | 230/441 | 52. 1 |
| Discoid lesions | 57/441 | 15.1 |
| Arthritis | 281/458 | 61.3 |
| Glomerulonephritis | 183/457 | 40.0 |
| Seizures | 48/457 | 10.5 |
| Psychosis | 23/455 | 5.0 |
| Serositis | 81/457 | 17.7 |
| Leukopenia | 136/455 | 29.8 |
| Lymphopenia | 80/450 | 17.7 |
| Hemolytic anemia | 39/460 | 8.4 |
| Thrombocytopenia | 97/460 | 21.08 |
| Anti Ro/SS-A | 161/441 | 36.5 |
| Anti La/SS-B | 80/440 | 18.1 |
| Anti RNP | 110/421 | 26.1 |
| Anti SM | 87/434 | 20.0 |
| Anti-dsDNA | 150/444 | 33.7 |
| Anticardiolipin IgG | 54/443 | 12.1 |
| Anticardiolipin IgM | 53/443 | 11.9 |
| Lupus anticoagulant | 59/407 | 14.4 |
| Rheumatoid factor | 95/411 | 23.1 |
| Antiphospholipid syndrome | 33/439 | 7.5 |
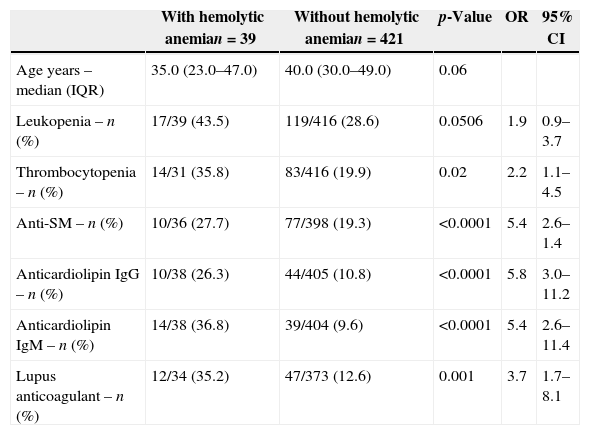
The comparison data of patients with and without hemolytic anemia (p-value <0.1) are shown in Table 2.
Association studies of demographic, clinical and serological variables of lupus patients with hemolytic anemia.
| With hemolytic anemian=39 | Without hemolytic anemian=421 | p-Value | OR | 95% CI | |
|---|---|---|---|---|---|
| Age years – median (IQR) | 35.0 (23.0–47.0) | 40.0 (30.0–49.0) | 0.06 | ||
| Leukopenia – n (%) | 17/39 (43.5) | 119/416 (28.6) | 0.0506 | 1.9 | 0.9–3.7 |
| Thrombocytopenia – n (%) | 14/31 (35.8) | 83/416 (19.9) | 0.02 | 2.2 | 1.1–4.5 |
| Anti-SM – n (%) | 10/36 (27.7) | 77/398 (19.3) | <0.0001 | 5.4 | 2.6–1.4 |
| Anticardiolipin IgG – n (%) | 10/38 (26.3) | 44/405 (10.8) | <0.0001 | 5.8 | 3.0–11.2 |
| Anticardiolipin IgM – n (%) | 14/38 (36.8) | 39/404 (9.6) | <0.0001 | 5.4 | 2.6–11.4 |
| Lupus anticoagulant – n (%) | 12/34 (35.2) | 47/373 (12.6) | 0.001 | 3.7 | 1.7–8.1 |
IQR: interquartile range; OR: odds ratio; 95% CI: 95% confidence interval.
Association studies of hemolytic anemia with disease duration, age at diagnosis, gender, photosensitivity, oral ulcers, malar rash, discoid lesions, arthritis, glomerulonephritis, seizures, psychosis, serositis, lymphopenia, anti-Ro/SS-A, anti-La/SS-B, anti-ribonucleoprotein (anti-RNP), anti-double stranded DNA (anti-dsDNA), rheumatoid factor and APS were not significant.
On further investigating variables with p-values <0.1 in univariate analysis using a logistic regression model, only anticardiolipin IgM remained significant [p-value=0.002; OR 5.1; 95% confidence interval (CI)=1.7–14.9].
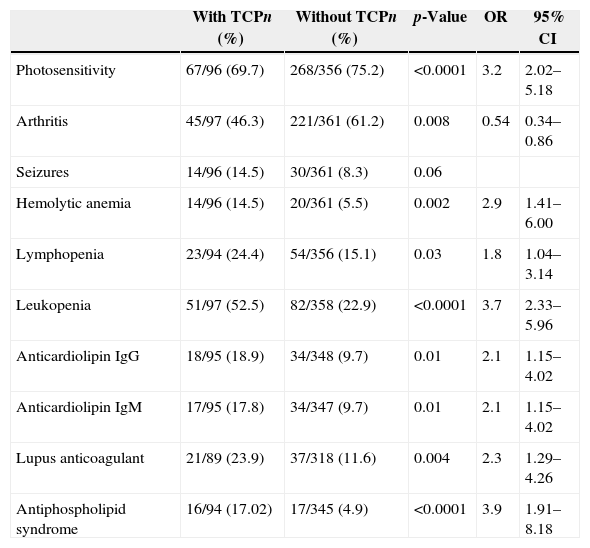
Association studies with leukopeniaData of patients with and without leukopenia (p-value <0.1) are listed in Table 3. Comparisons of age, disease duration, age at diagnosis, gender, photosensitivity, oral ulcers, malar rash, discoid lesions, arthritis, glomerulonephritis, serositis, presence of anti-Ro/SS-A; Anti-La/SS-B, anti-RNP, anticardiolipin IgG and IgM, rheumatoid factor and APS were not significant.
Comparison of lupus patients with (n=136) and without (n=319) leukopenia.
| With leukopenian (%) | Without leukopenian (%) | p-Value | OR | 95% CI | |
|---|---|---|---|---|---|
| Seizures | 20/136 (14.7) | 28/316 (8.8) | 0.06 | ||
| Psychosis | 14/136 (10.2) | 9/317 (2.8) | 0.0009 | 3.9 | 1.6–9.3 |
| Lymphopenia | 34/133 (25.5) | 46/315 (14.6) | 0.005 | 2.00 | 1.21–3.31 |
| Hemolytic anemia | 17/135 (12.5) | 22/319 (6.8) | 0.04 | 1.9 | 0.99–3.7 |
| Thrombocytopenia | 51/136 (37.5) | 46/317 (14.5) | <0.0001 | 3.5 | 2.2–5.6 |
| Anti-dsDNA | 55/134 (41.0) | 95/307 (30.9) | 0.03 | 1.55 | 1.02–2.36 |
| Lupus anticoagulant | 27/123 (21.9) | 32/283 (11.3) | 0.005 | 2.20 | 1.25–3.87 |
OR: odds ratio; 95% CI: 95% confidence interval.
On including the variables with a p-value <0.1 in the univariate analysis in a logistic regression model, lymphopenia (p-value=0.02; OR=1.8; 95% CI=1.06–3.15), psychosis (p-value=0.01; OR=3.1; 95% CI=1.22–8.03); thrombocytopenia (p-value <0.0001; OR=3.2; 95% CI=1.93–5.33); and anti-dsDNA (p-value=0.03; OR=1.6; 95% CI=1.02–2.54) were independently associated with leukopenia.
Association studies of thrombocytopeniaAssociation studies of thrombocytopenia are shown in Table 4. Comparative analysis of associations with age, disease duration, age at diagnosis, gender, oral ulcers, malar rash, discoid lesions, glomerulonephritis, psychosis, serositis, anti-Ro/SS-A, anti-La/SS-B, anti-RNP, anti-SM, anti-dsDNA and rheumatoid factor were found to be non-significant.
Comparison of lupus patients with (n=97) and without (n=363) thrombocytopenia.
| With TCPn (%) | Without TCPn (%) | p-Value | OR | 95% CI | |
|---|---|---|---|---|---|
| Photosensitivity | 67/96 (69.7) | 268/356 (75.2) | <0.0001 | 3.2 | 2.02–5.18 |
| Arthritis | 45/97 (46.3) | 221/361 (61.2) | 0.008 | 0.54 | 0.34–0.86 |
| Seizures | 14/96 (14.5) | 30/361 (8.3) | 0.06 | ||
| Hemolytic anemia | 14/96 (14.5) | 20/361 (5.5) | 0.002 | 2.9 | 1.41–6.00 |
| Lymphopenia | 23/94 (24.4) | 54/356 (15.1) | 0.03 | 1.8 | 1.04–3.14 |
| Leukopenia | 51/97 (52.5) | 82/358 (22.9) | <0.0001 | 3.7 | 2.33–5.96 |
| Anticardiolipin IgG | 18/95 (18.9) | 34/348 (9.7) | 0.01 | 2.1 | 1.15–4.02 |
| Anticardiolipin IgM | 17/95 (17.8) | 34/347 (9.7) | 0.01 | 2.1 | 1.15–4.02 |
| Lupus anticoagulant | 21/89 (23.9) | 37/318 (11.6) | 0.004 | 2.3 | 1.29–4.26 |
| Antiphospholipid syndrome | 16/94 (17.02) | 17/345 (4.9) | <0.0001 | 3.9 | 1.91–8.18 |
TCP: thrombocytopenia; OR: odds ratio; 95% CI: 95% confidence interval.
When the variables with p-values <0.1 in univariate analysis were assessed using logistic regression, arthritis remained inversely associated to thrombocytopenia (OR=0.3; 95% CI=0.20–0.61) and leukopenia (OR=3.1; 95% CI=1.82–5.44) and APS (OR=3.1; 95% CI=1.28–7.87) were associated to thrombocytopenia.
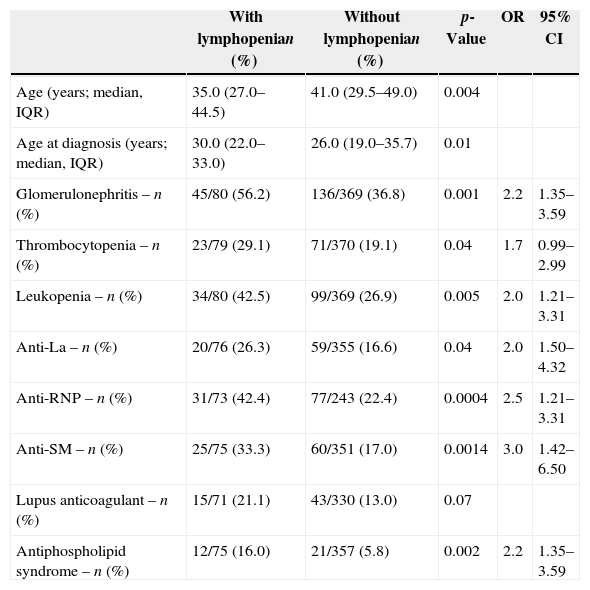
Association studies of lymphocytopeniaAssociations of variables with lymphocytopenia are shown in Table 5. Analysis of disease duration, gender, photosensitivity, oral ulcers, malar rash, discoid lesions, arthritis, seizures, psychosis, serositis, hemolytic anemia, anti-Ro, anti-dsDNA, anticardiolipin IgG and IgM and rheumatoid factor were non-significant.
Comparison of lupus patients with (n=80) and without (n=370) lymphopenia.
| With lymphopenian (%) | Without lymphopenian (%) | p-Value | OR | 95% CI | |
|---|---|---|---|---|---|
| Age (years; median, IQR) | 35.0 (27.0–44.5) | 41.0 (29.5–49.0) | 0.004 | ||
| Age at diagnosis (years; median, IQR) | 30.0 (22.0–33.0) | 26.0 (19.0–35.7) | 0.01 | ||
| Glomerulonephritis – n (%) | 45/80 (56.2) | 136/369 (36.8) | 0.001 | 2.2 | 1.35–3.59 |
| Thrombocytopenia – n (%) | 23/79 (29.1) | 71/370 (19.1) | 0.04 | 1.7 | 0.99–2.99 |
| Leukopenia – n (%) | 34/80 (42.5) | 99/369 (26.9) | 0.005 | 2.0 | 1.21–3.31 |
| Anti-La – n (%) | 20/76 (26.3) | 59/355 (16.6) | 0.04 | 2.0 | 1.50–4.32 |
| Anti-RNP – n (%) | 31/73 (42.4) | 77/243 (22.4) | 0.0004 | 2.5 | 1.21–3.31 |
| Anti-SM – n (%) | 25/75 (33.3) | 60/351 (17.0) | 0.0014 | 3.0 | 1.42–6.50 |
| Lupus anticoagulant – n (%) | 15/71 (21.1) | 43/330 (13.0) | 0.07 | ||
| Antiphospholipid syndrome – n (%) | 12/75 (16.0) | 21/357 (5.8) | 0.002 | 2.2 | 1.35–3.59 |
IQR: interquartile range; OR: odds ratio; 95% CI: 95% confidence interval.
In the logistic regression study of variables with p-values <0.1 in the univariate analysis, only leukopenia (OR=1.8; 95% CI=1.01–3.29) and lupus anticoagulant (OR=2.2; 95% CI=1.16–4.39) remained independently significant.
DiscussionHematological findings in lupus patients are very common and may be the presenting feature of the disease. In the current study hemolytic anemia was the least common manifestation (8%) followed by lymphopenia (18%), thrombocytopenia (21%) and leukopenia (30%).
There was an association between hemolytic anemia and anticardiolipin IgM antibodies; this association has been described in other studies. Lang et al.20 described associations with both anticardiolipin IgG and IgM antibodies. Sultan et al.,18 studying 305 lupus patients from the United Kingdom, found an association with anticardiolipin IgG antibodies but Deleze et al.21 studying Spanish lupus patients and Cervera et al.8 analyzing a Mexican sample reported strong associations with anticardiolipin IgM antibodies similar to the current study.
Leukopenia was the most common hematological finding in this study appearing in almost one in three of the patients. The importance of this finding is highlighted when one notes that infections are a leading cause of death in SLE patients.22 Bacterial infections are the most common, followed by viral and fungal infections.22 In this sample, leukopenia was associated with lymphopenia, psychosis, thrombocytopenia and anti-dsDNA. The correlation between this finding, lymphopenia and ds-DNA has been reported by others.17 A low lymphocyte count is found to be independent of (although contributory to) leukopenia17 and has been associated, in the literature, to higher lupus activity,23 more severe acral damage,23 and some clinical disease characteristics such as neurologic involvement.17 In the current sample, although lymphopenia was found to be associated with glomerulonephritis, thrombocytopenia, anti-RNP, anti-SM, APS, lupus anticoagulant and leukopenia, only the last two remained significant after logistic regression. Lupus disease activity and cumulative damage were not studied.
SLE thrombocytopenia results from disease activity or from suppression of the bone marrow by an immunosuppressant.24 Autoantibodies against platelets, against thrombopoietin and bone marrow abnormalities have been detected in these patients.24 Although antibodies against platelets are common among thrombocytopenic patients they are not always linked to low platelet counts.24 Furthermore, anti-thrombopoietin autoantibodies are considered to have a weak effect on platelet counts.24 In the current study positive associations were found for thrombocytopenia with APS and with leukopenia. The association between APS and thrombocytopenia is well known not only in lupus but in other autoimmune thrombocytopenias.17
ConclusionThe most common hematological abnormality of the SLE classification criteria in a cohort of Brazilian SLE patients was leukopenia followed by thrombocytopenia, lymphopenia and hemolytic anemia. Low platelet counts and hemolysis were associated to APS and anticardiolipin IgM, respectively. Leukopenia and lymphocytopenia are correlated and leukopenia is more common in SLE patients with psychosis, thrombocytopenia and anti-dsDNA.
Conflicts of interestThe authors declare no conflicts of interest.