Allogeneic hematopoietic cell transplantation (HCT) is a curative treatment for several benign and malignant diseases. 1 Chronic graft-versus-host disease (cGVHD) is one of the main causes of mortality and morbidity after HCT. Its immunopathophysiology is complex and involve changes in B and T cells, activation of innate immune cells such as macrophages, dendritic cells, and neutrophils that activate inflammatory pathways.2
The bacterial genus Mycoplasma belongs to the class of the smallest free-living replicant Tenericutes. Their main habitats in humans are respiratory and the urogenital tract. Its size is extremely small, varying from 0.2 to 0.8 µm in diameter.3 The absence of cell walls and its poor genome (only 580–1380 kb) allows the occurrence of mutations and antigenic variations that favor the development of antibiotic-resistant strains. It has extremely slow growth, even under appropriate conditions what make it even more difficult to identify. Culture is the gold standard test but it can require up to 28 days to view colonies.4
Mycoplasma salivarium (Ms) is an oral cavity commensal pathogen present mainly in the bacterial plaque and gingival sulcus. It is present in the amount of about 10 genome copies per microliter in normal oral tissue.5 This species is associated with infectious processes, especially in immunosuppressed individuals, and is also considered a stimulating factor for chronic inflammatory conditions.6 There are no reports in the literature about oral infection by Ms in recipients of HCT or about its relationship with cGVHD. The objective of this study is to report a case of oral mucosa infection by Mycoplasma salivarium in a patient with chronic graft-versus-host disease.
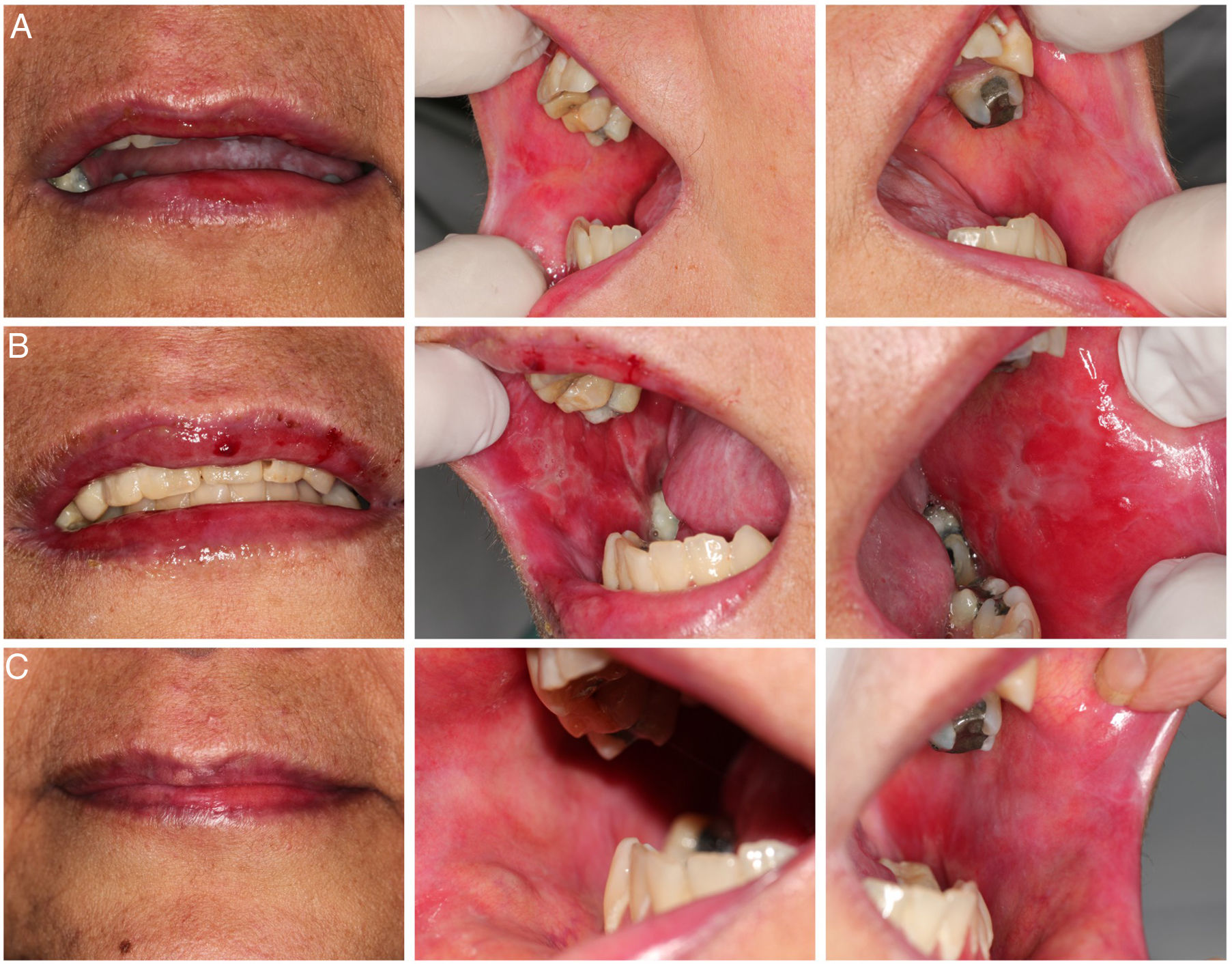
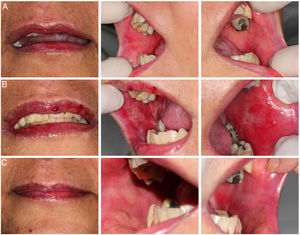
Case reportThe patient was a 47-year-old woman who underwent allogeneic HCT related to myelodysplastic syndrome. Conditioning was with fludarabine 50 mg and bussulfan 61 mg both for 4 days and prophylaxis for GVHD with 72 mg of methotrexate. Thirty days after cell infusion (D + 30), she developed grade II acute GVHD in the skin and gastrointestinal tract that was treated with high doses of prednisone (60 mg every 12 h) until D + 100, showing a complete response. At day +240 post cell infusion, she developed grade II classic chronic GVHD affecting mouth, eyes, and skin, treated with prednisone 20 mg per day and methotrexate 15 mg per week in an alternate protocol, with a partial response and prednisone was maintained in a reduced dose (10 mg per day). Since then, GVHD in the oral mucosa had remained with stable lichenoid lesions and without symptoms. (Fig. 1-A). Three years after cell infusion (D + 1096), she attended the Dentistry and Stomatology Service at the Clinical Hospital of Ribeirão Preto-USP with a complaint of “burning and bleeding in the oral cavity, with difficulty feeding, lasting approximately 5 days”.
On clinical examination, hemorrhagic spots were observed with an ulcerated area in the upper lip vermilion; erythematous, erosive, and ulcerated lesions in the bilateral bucal mucosa (Fig. 1-B). It was also observed important biofilm accumulation on teeth surface but no signal of periodontal bone loss or caries. At this moment, the patient was using the following medications: prednisone (10 mg/day), acyclovir (200 mg/2x a day), and sulfamethoxazole (400 mg) + trimethoprim (80 mg) 2x a day/2x a week. The patient did not show signs of GVHD complications in other organs. The main diagnostic hypotheses were infection, overlap of acute GVHD and drug toxicity. A biopsy for histopathological examination; staining for fungi and alcohol-resistant bacteria; immunohistochemistry for viruses; PCR (Polymerase Chain Reaction) for herpes virus types I and II, Cytomegalovirus, Epstein-Barr virus; and culture for fungi were performed. The material adhered to the lesions was also collected with a swab for culture of fungi and bacteria. As patient had no systemic involvement at this time only analgesic was prescribed and the patient was monitored until results.
The histopathological samples analysis identified acute mucositis with lymphomononuclear inflammatory infiltrate on the surface of the tissue and in the chorion and perivascular infiltrate, without characteristics for GVHD or drug toxicity. As the patient presented changes only in the oral cavity and no medication had been introduced in recent days, the main diagnostic hypothesis was infection. The other tests performed from the biopsy were negative for bacterial, viral, and fungal structures. The bacterial culture showed the presence of Streptococcus aureus sensitive to erythromycin, which was prescribed on day + 2006 (10 days after initial dentistry appointment).
After 7 days of erythromycin use patient did not show improvement of oral signs and symptoms and remain without other organs or systemic complications. In face of negative results for other infectious agents we decided to investigate infection for Mycoplasm spp because of its difficulty to recover by conventional culture and previous description of infection in the oral cavity of immunosuppressed patients.3,5,6 While waiting the results it was maintained erythromycin use and monitoring.
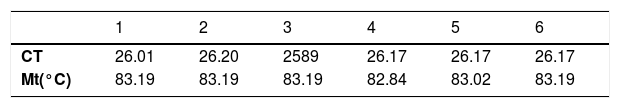
From a new biopsy, genetic material (DNA) was obtained to perform real-time PCR amplification of the 16S rDNA gene with BRYT Green® double-stranded fluorescent DNA intercalant (GoTaq®qPCR Master Mix, Promega). All of six replicates showed positive result with amplification of the target DNA sequence. The means of the amplification cycle threshold (Ct) and melting temperature (MT) were 26.10 (Ct @ 26) and 83.10 °C, respectively (Table 1).
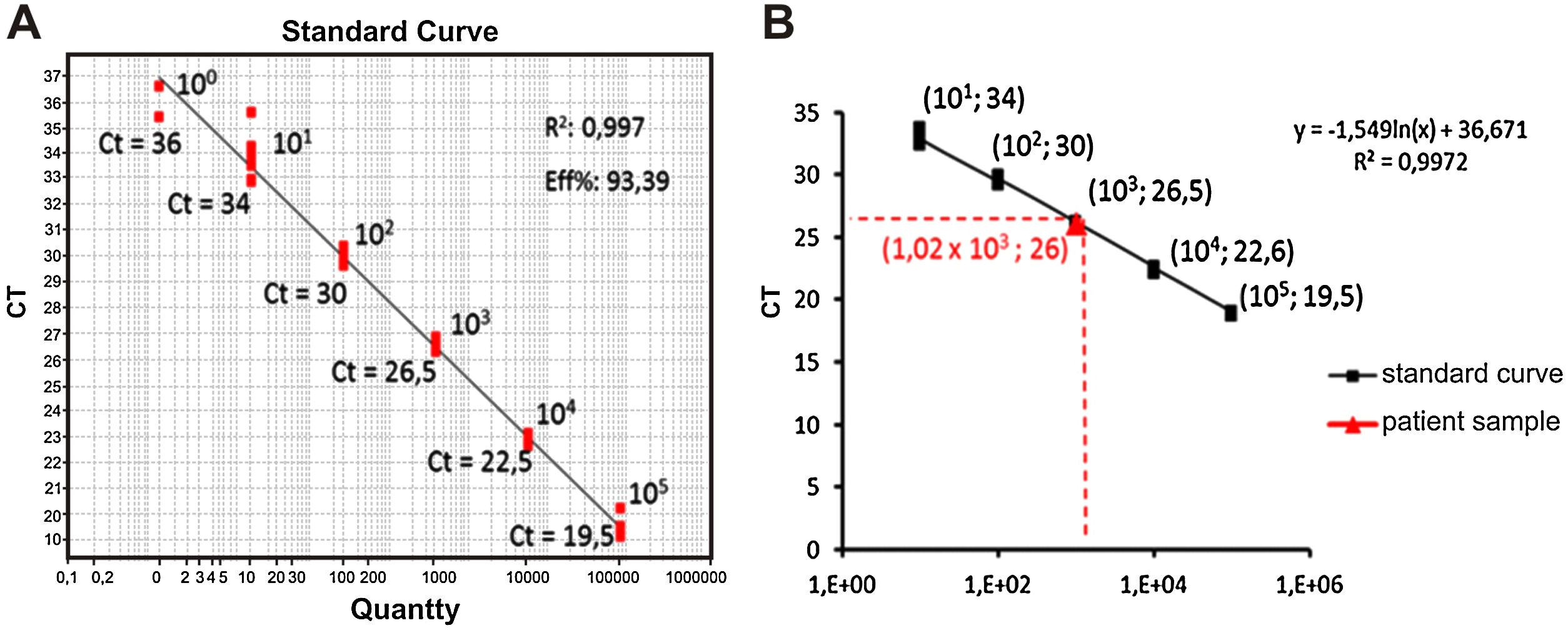
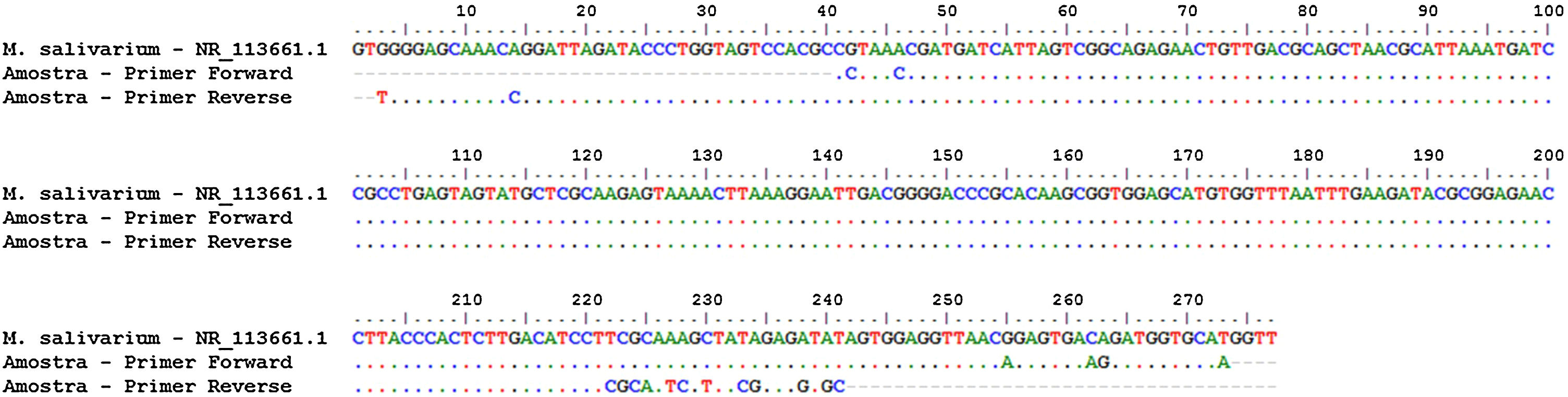
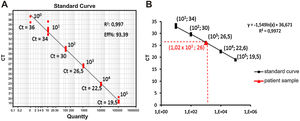
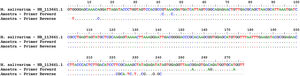
In order to calculate the number of copies of the bacterial genome in the sample, the average value of Ct (Ct ≅ 26) was inserted in the predetermined equation of the line (y = -1.54 ln (x) + 36.67; where y = Ct) of the standard test curve, thus determining the presence of 1.02 × 103 copies of the genome/µL of sample (Fig. 2). To identify the Mycoplasma species, the genetic material was sequenced (ABI 3500xL Genetic Analyzer). The consensus sequences were assembled using the BioEdit Sequence Alignment Editor version 7.0.9.0 (Ibis Biosciences), and these were analyzed using the Basic Local Alignment Search Tool (BLAST) algorithm, which showed 99% genetic identity with the species Mycoplasma salivarium (accession number NR_113661.1) (Fig. 3).
Determination of the number of copies of the bacterial genome in the test sample. (A) Positive control: Standard curve of the molecular test, points identified with their respective amplification Ct and number of copies of the Mycoplasma genome, (B) Calculation by linear regression to obtain the number of copies of the Mycoplasma genome present in the test sample.
Sequencing and identification of Mycoplasma species. Alignment: sequence of the 16S rDNA gene of Mycoplasma salivarium (NR_113661.1) with the sequences obtained from sequencing of the test sample carried out with forward and reverse primers. Dotted lines indicate genetic homogeneity between the sequences analyzed.
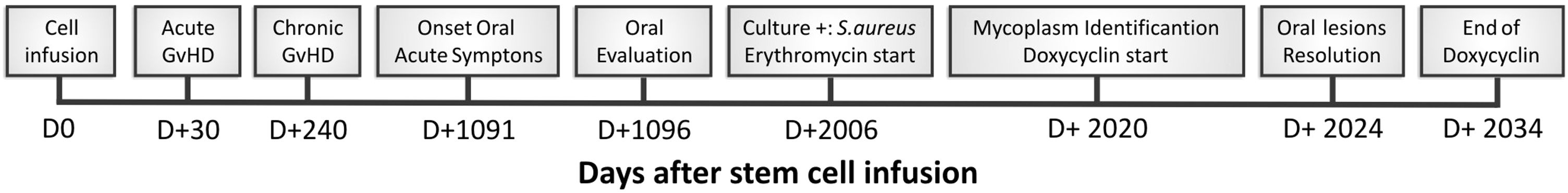
Based on the findings, on day +2020 (24 days after dentistry initial appointment) treatment was started with oral doxycycline 200 mg/day. Four days after, oral lesions completely regressed (Fig. 1-C) and doxycycline was maintained until complete 14 days of treatment (D + 2034 post stem cell infusion). In the end of doxycycline use, a new biopsy and a new real-time PCR were performed, with negative results for Mycoplasma spp. Fig. 4 represents the timeline for the case.
DiscussionAlthough it is considered a commensal pathogen residing mainly in the bacterial plaque and gingival sulcus of healthy individuals, the concentration of Ms appears to be higher in individuals with periodontal disease. But there is no well-established cause-and-effect relationship.5 In a more recent study, Sugiyama6 suggested that Ms is capable of activating several inflammatory proteins, including NLRP3 and THP-1 in monocytic cells, inducing the production of IL-1β. Thus, an association of this organism with the inflammatory process of periodontal disease may be suggested. There are few publications evaluating the clinical repercussions of Ms. Among those found in research databases of the last 30 years, a higher concentration of Ms in the saliva of HIV-positive patients,7 a case of a submassetic abscess of dental origin caused by Ms,8 a case of arthritis in a patient with hypogammaglobulinemia,9 a case of septic arthritis by Ms in a patient with chronic lymphoid leukemia,10 Ms infection in an orthopedic prosthesis,11 empyema caused by Ms12 and two cases of Ms isolated from brain abscess13 have been described. Similarly, all reported cases have in common the diagnostic difficulty.
Ms is a challenge to identify due to its small size and slow growth in routine culture media, which is why it is speculated that there is an underdiagnosis of infections caused by Ms. In this scenario, patients undergoing allogeneic HCT, especially those with long-term immunosuppression are considered an important risk group. The correlation between intestinal GVHD and the local microbiota seems to be well established.14 However, there are no studies that assess this correlation with the oral cavity.In addition, hyposalivation, associated with poor oral hygiene and immunosuppression, are risk factors for the development of infection in the periodontium and oral mucosa.15
Lichenoid reactions in the oral mucosa are the classic manifestation of chronic GVHD. Erythema and ulceration are among the distinguishing lesions in cGVHD and can overlap the lichenoid condition. So these manifestations are often treated as cGVHD. In our case, it was noteworthy that the patient had a long-term stable cGVHD and acute manifestation only in the oral mucosa. As described above, we had difficulties identifying Ms as a causal agent, which was only possible due to the availability of the quantitative PCR test in our service. As no antimicrobial sensitivity test was performed, we chose to treat it based on descriptions in the literature. The clinical response obtained, combined with the negative PCR result of after finishing the doxycycline use, seems to us an unequivocal condition of the relationship between the microorganism and the local inflammatory condition.
An incorrect diagnosis and treatment for cGVHD in the face of an infectious condition can have unexpected consequences not only in terms of local complications but also in the risk of disease relapse related to systemic immunosuppression. In our case, if the diagnosis were cGVHD, the probable approach would be to increase systemic immunosuppression. This could bring a temporary local response, as it acts in the inflammatory condition, but it would not deal with the factor that triggered the condition.
We can conclude that the differential diagnosis between GVHD and infectious complications in the oral cavity can be critical and that, in suspected cases, the investigation should be carried out carefully, including for apparently rare conditions such as infections by Ms. There is a need for studies that correlate the microbiota with the development and maintenance of oral cGVHD.
Conflict of interestThe authors declare no conflicts of interest.
We thank the dentists, medical and nursing staff of Ribeirão Preto Clinical Hospital for their support. We received no financial support.